UDC 577.1:616.89
ENDOGENOUS ETHANOL AND ACETALDEHYDE,
THEIR BIOMEDICAL SIGNIFICANCE (Literature Review)
Yu. A. Tarasov, Ph.D. sci., senior researcher; V. V. Lelevich, Doctor of Medical Sciences, Professor
Educational Establishment "Grodno State Medical University"
The review presents literature data on the metabolism of endogenous ethanol and acetaldehyde in the body, as well as their biological significance.
Keywords: endogenous ethanol, acetaldehyde, alcohol dehydrogenase, aldehyde dehydrogenase, pyruvate dehydrogenase.
The review presents the literature data on the metabolism of endogenous ethanol and acetaldehyde in the organism, as well as their biological value.
Key words: endogenous ethanol, acetaldehyde, alcohol dehydrogenase, acetaldehyde dehydrogenase, pyruvate dehydrogenase.
When characterizing the biological activity of ethanol and its metabolite, acetaldehyde, two aspects of the problem should be emphasized. First, when it comes to these compounds, as natural metabolites, constantly (endogenously) present in the body in physiological concentrations. Secondly, when a situation arises with the exogenous intake of alcohol into the body, that is, the formation of states of acute or chronic alcohol intoxication.
Ethanol and its metabolites are natural components of metabolism and are indispensable participants in homeostatic mechanisms. To assess the metabolic significance of endogenous ethanol, its level in blood and tissues should be compared with the content of known substrates - participants in metabolism in humans and animals (see table). This makes it possible to make sure that, taking into account the relatively small molecular weight of ethanol, it is easily placed on a par with the intermediate products of carbohydrate and protein metabolism. From the data presented in the table, it follows that several orders of magnitude lower than endogenous ethanol, in this series is the concentration of the neurotransmitter. But the content of acetaldehyde, which is constantly present in the body in equilibrium (1:100) ratios with ethanol, is quite comparable with it. This suggests that the role of the ethanol/acetaldehyde pair in maintaining homeostatic metabolic functions is similar to that performed in the body by the glucose/glucose-6-phosphate and lactate/pyruvate ratios in controlling glycolysis reactions and stabilizing the levels of glycolysis intermediates.
The amount of pyruvate in tissues is 2-3 orders of magnitude lower than that of lactate, but pyruvate itself, like acetaldehyde, is highly reactive. Under changing metabolic situations, pyruvate levels shift significantly
Compound Blood (mol/l) Liver (mol/kg)
Glucose 5 - 10 - 3
Glucose-6-phosphate 2 ■ 10- 4
Fructose-6-phosphate 2■10-4
Phosphodioxyacetone 10- 5 - 10- 4 10-4
Amino acids 10-4 - 10-3
Ethanol 10- 4 10- 4
Adrenaline 10-9
to a lesser extent than the level of lactate, which undoubtedly reflects the greater importance in the metabolism of the first, rather than the second, compound. Therefore, lactate is regarded as a buffer metabolic impasse, leveling fluctuations in pyruvate. From the same standpoint, the ethanol/acetaldehyde system is a similar checkpoint for two-carbon compounds and acetaldehyde itself. This assessment of the ethanol/acetaldehyde relationship quite satisfactorily explains the lability of the endogenous ethanol level under a variety of exposures. Thus, endogenous ethanol plays the role of a buffer that is in equilibrium dynamic relationships with its very active precursor, acetaldehyde. The β-ethanol/acetaldehyde pair under consideration (see the figure) performs similar functions of a buffer pool with respect to the very active metabolite α-acetaldehyde, especially with respect to neurohormones. Ethanol works in this system as a buffer reserve for acetaldehyde, leveling the fluctuations that inevitably arise due to the sinusoidal nature of the flow of multi-link chain reactions in metabolism.
Carbohydrates, lipids, amino acids
Lactate □ pyruvate □ acetyl-CoA
Ethanol □ acetaldehyde □ acetate
Other sources
Figure - Lactate and ethanol as metabolic "dead ends" in the exchange of pyruvate and acetaldehyde
The heterogeneity of the functions of endogenous ethanol, which can be very different - an energy source, a precursor of acetaldehyde, which is involved in the synthesis of endogenous morphine-like compounds, and which is the strongest modifier of amine and sulfhydryl groups in proteins. Acetaldehyde, as the most powerful modifier of proteins, changes not only their reactivity, but also spatial characteristics, i.e., the parameters that are most important for the effective binding of neurotransmitters by receptor proteins. The amphiphilic nature of ethanol and acetaldehyde plays a significant role in maintaining a certain hydrophobicity of proteins and the necessary functional fluidity of the latter.
Both compounds are considered as two-carbon radicals capable of competitively interacting with many other two-carbon molecules at the level of active sites of enzymes, transport proteins, and specific receptors. The membranotropism of ethanol is functionally important in the pathogenesis of the manifestations of alcoholic disease, since various diols, moreover, which do not form acetaldehyde, can relieve the manifestations of ethanol withdrawal syndrome. The ethanol/acetaldehyde pair may be of particular importance in relationships with neurotransmitters containing hydroxyl or carbonyl groups, hormones, their precursors, and metabolites, since the concentration of these bioregulators is significantly lower than the concentration of endogenous ethanol and acetaldehyde.
The amount of endogenously formed and metabolized acetaldehyde and ethanol, therefore, should be considered as a factor that controls a significant part of the homeostatic mechanisms that ultimately form the state that any organism always strives for - "metabolic comfort".
Repeatedly repeated in different seasonal periods of the year, the selection of animals in relation to the consumption of ethanol solutions always made it possible to isolate from the general population of rats that prefer water (W) or ethanol (E). PE accounted for approximately 5-10% of all animals tested. A distinctive feature of PE individuals was that the content of endogenous ethanol in the blood, and especially in the liver, was always 2-3 times lower than in PV. In turn, the found inverse correlation relationships between the level of endogenous ethanol and voluntary alcohol consumption essentially repeat the pathogenetic situation: the value of endogenous ethanol and acetaldehyde is such that when they are deficient in the body, additional alcohol intake becomes the simplest way of self-correction. In turn, the extrapolation of these relationships to the mechanisms of the pathogenesis of alcoholism makes it possible to believe that prolonged excessive alcohol consumption, forced in experiments on animals and voluntary or socially motivated in humans, eventually replacing the production of endogenous ethanol and acetaldehyde, first leads to inhibition, and then to degradation of systems of endogenous synthesis of these compounds. That is, to a situation where the external intake of alcohol into the body becomes necessary. To a large extent, naturally, simplified, without taking into account the drug addiction factor in pathogenesis, such relationships can explain the phenomenon of physical dependence, as well as an understanding of why, in delirious states, the best and easiest way to stop them is to introduce alcohol to the patient.
The relationship between alcohol motivation and the level of endogenous ethanol can also be traced in other experimental situations. Thus, various factors affecting the consumption of alcohol by animals or drugs used for treatment, according to the effect on the level of endogenous ethanol in the blood and liver, were divided into two diametrically opposed groups. All effects that enhance alcohol motivation, such as: stress, starvation, oxythiamine, iproniazid, tetrahydroisoquinolines - reduce, and weakening alcohol motivation (thiamine, thiamine diphosphate, riboflavin, diethyldithiocarbamate, glutamine, lithium chloride) -
raise the level of endogenous ethanol. These data are supplemented by studies by other authors in relation to tranquilizers, castration, and experiments in which rats differently sensitive to the narcotic effect of ethanol also differed in the level of endogenous ethanol. Determining the level of endogenous ethanol is used in narcological clinics in Poland for dynamic control of the applied therapeutic treatment of patients with alcoholic disease. In the clinic for the treatment of alcohol dependence of the St. Petersburg Psychoneurological Institute. V. M. Bekhterev successfully used a method for the treatment of alcoholism, based on the restoration of homeostasis of endogenous ethanol in the body of patients.
It should be noted that the listed variants of the manifestation of the activity of ethanol and acetaldehyde are important not only in acute and chronic alcohol intoxication, but, which is paramount, in natural conditions, with the endogenous functioning of the compounds. At the same time, two options are distinguished in the assessment of the biological activity of ethanol: metabolic and toxicological. In the first case, endogenous ethanol is at the head - as a natural metabolite of metabolism. In the second case, ethanol that enters the body in excess acts as a powerful toxicological agent and a factor in the metabolic disintegration of metabolism. Both in one and in the other case, practically the same systems work that metabolize alcohol and aldehyde, and all the main systems of the body are included in the processes of metabolism of these compounds. Alcohol entering the body is oxidized by 75-95% in the liver. Other organs have a much lower ability to metabolize ethanol. In addition, small amounts are excreted from the body with urine and exhaled air.
The main alcohol-metabolizing systems:
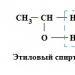
Alcohol dehydrogenase (ADH, EC 1.1.1.1) is an enzyme widely distributed in animal tissues and plants. ADH catalyzes the reversible conversion of alcohols to the corresponding aldehydes and ketones with NAD as a cofactor:
Alcohol + NAD □ aldehyde + NADH + H+
It should be emphasized that at physiological pH, the reduction of aldehydes or ketones proceeds ten times faster than the oxidation of alcohols. Only with a multiple (100-1000 times) increase in the concentration of ethanol, as happens when the body is loaded with alcohol, the enzyme functions in the opposite direction. Substrates for ADH are primary and secondary aliphatic alcohols and aldehydes, retinol, other polyene alcohols, diols, pantothenyl alcohol, steroids, □-hydroxy fatty acids, 5-hydroxyethylthiazole, and others. Moreover, it should be noted that ethanol and acetaldehyde are not the best substrates for ADH. The study of the intracellular distribution of ADH in the liver showed that the enzyme is localized in the cytosol of hepatocytes, but not in Kupffer cells. The great functional significance of ADH is confirmed by changes in the activity of the enzyme in organs and tissues under various pathological conditions. A natural function of ADH, present in vast quantities in the liver of humans and animals, is that the enzyme produces rather than consumes endogenous ethanol and thus actively regulates its levels and maintains endogenous acetaldehyde homeostasis.
Microsomal ethanol-oxidizing system (MEOS). The oxidation of ethanol by microsomes proceeds according to the following equation:
С2Н5ОН + NAPH + Н+ + О 2 □ CH 3СНО + NADP+ + 2Н О The optimum pH of this reaction lies in the physiological region, KM for ethanol is 7-10 Mm, which is much higher than for ADH. MEOS differs from ADH and catalase in sensitivity to inhibitors, as well as in a number of other properties. It is insensitive to the action of pyrazole and sodium azide. MEOS is activated by propylthiouracil and thyroid hormones. It is believed that MEOS is identical with nonspecific oxidases that detoxify drugs in the liver, and that it is through MEOS that the ADH-independent pathway of ethanol oxidation in the body of mammals passes. MEOS, with all evidence, functions independently of ADH and catalase, and its contribution to the oxidation of ethanol is normally about 10%, but significantly increases with alcohol intoxication.
Catalase (EC 1.11.1.6) in the presence of hydrogen peroxide is able to oxidize ethanol to acetaldehyde according to the equation:
C COH + CO2 □ CH3CHO + 2H2O The enzyme functions in a wide range of animal tissues, and has both species and individual fluctuations in its activity. Sources of hydrogen peroxide are reactions catalyzed by glucose oxidase, xanthine oxidase, NADPH oxidase. The maximum activity of catalase occurs at physiological pH. The rate of the catalase reaction depends on the concentration of ethanol and the rate of formation of hydrogen peroxide. The body has a significant number of systems that generate hydrogen peroxide and are localized in peroxisomes, endoplasmic reticulum, mitochondria, cytosol and create a concentration of hydrogen peroxide in the range of 10-8 - 10-6M. Like MEOS, the catalase pathway of ethanol oxidation is classified as a minor one, which acquires a certain significance only at high concentrations of ethanol in the body or under conditions of ADH inhibition.
The possibility of ethanol oxidation by transferring its molecule to the □-hydroxyethyl radical was shown, which can occur when electrons are transferred by nitric oxide synthase, which is capable of forming a superoxide radical, as well as hydrogen peroxide. Researchers express the opinion that nitric oxide synthase in terms of the level of ethanol oxidation is no less significant than cytochrome P-450, provided that L-arginine is present as the main substrate.
One of the sources of endogenous ethanol in the animal body is the intestinal microflora. In experiments on angiostomized animals, by simultaneous sampling of blood from the portal vein and the peripheral venous bed, it was shown that the blood flowing from the intestine contains more ethanol than the blood flowing from the liver.
When assessing the balance relations in the exchange of ethanol, therefore, one should take into account its two sources and the main, decisive role of hepatic alcohol-gold dehydrogenase in the regulation of the level of alcoholemia.
Oxidation of aldehydes in the body of mammals occurs mainly by non-specific aldehyde dehydrogenase (AlDH, K.F.1.2.1.3). The reaction catalyzed by an enzyme is irreversible:
CH3CHO + NAD+ + H2O □ CH 3COOH + NADH + 2H+
Liver aldehyde dehydrogenases are represented by two enzymes: with low (high Km) and high (low Km) affinity for acetaldehyde, preferably using aliphatic substrates and NAD as a coenzyme or aromatic aldehydes and NADP as a coenzyme. AlDH exists in multiple molecular forms differing in structure, catalytic characteristics, and subcellular localization. In mammals, AlDH isoenzymes are classified into five different classes. Each class has a specific cellular localization that is prevalent in different species, suggesting a very early divergence in the evolution of AlDH. In addition to dehydrogenase, liver AlDH has esterase activity. AlDH activity has been found in mitochondria, microsomes, and cytosol.
Known, but less studied, are other enzymes involved in the conversion of acetaldehyde, such as: aldehyde reductase, aldehyde oxidase and xanthine oxidase. But, as noted above, the reduction of acetaldehyde in the body is carried out mainly by AlDH, and so far, acetaldehyde is considered the only known precursor of endogenous ethanol.
For animal tissues, the following enzymes are known to be involved in the production of acetaldehyde:
Pyruvate dehydrogenase (EC 1.2.4.1) usually catalyzes the oxidative decarboxylation of pyruvate to acetyl-CoA. At the same time, the decarboxylating component of this polyenzymatic complex is capable of releasing free acetaldehyde during the reaction. The latter is either oxidized by AlDH in mitochondria to acetate, or ADH is reduced to ethanol in the cytoplasm.
O-phosphorylethanolamine phospholiase (EC 4.2.99.7)
An enzyme that breaks down phosphoethanolamine to acetaldehyde, ammonia and inorganic phosphate.
Threoninaldolase (K.F.4.1.2.5) - catalyzes the reaction of cleavage of threonine to glycine and acetaldehyde.
Aldolase (EC 4.1.2.7) of animal tissues has specificity only in the binding of dihydroxyacetone phosphate and uses any aldehydes as a second substrate. In turn, in the reverse reaction, acetaldehyde is formed in this way.
Recently, it has been shown that a decrease in the concentration of acetaldehyde in animal tissues, under conditions of selective inhibition of the activity of pyruvate dehydrogenase, can be counteracted by the inverse nature of changes in the activity of phosphoethanolamine lyase and threonaldolase.
It is also known that during the decomposition of □-alanine, a degradation product of pyrimidine nitrogenous bases, malonic aldehyde is first formed, and then acetaldehyde.
Concluding the analysis of the literature data, it should be noted that endogenous ethanol is constantly present in the human and animal organisms at concentrations comparable to the levels of other natural intermediates.
metabolic diatoms. The level of endogenous ethanol in the blood and tissues is modulated by various compounds (hormones, vitamins, antimetabolites, amino acids and their derivatives, lithium salts, disulfiram, cyanamide) and changes under various functional states of the body (stress, starvation, aging), the mechanism of action of which is clearly heterogeneous. The very balance in the endogenous ethanol/acetaldehyde system, provided by ADH and other enzymes that produce and consume acetaldehyde, obviously controls both the exchange of two-carbon and the synthesis of morphine-like compounds, regulates the activity of some neurotransmitters, peptides and proteins. In turn, changes in the activity of alcohol- and aldehyde-metabolizing systems, both under their physiological conditions and under conditions changed by alcohol loads, are essentially adaptive, providing the appropriate functional and metabolic homeostasis.
The review is dedicated to the blessed memory of the Teacher, Academician Yuri Mikhailovich Ostrovsky, who made a significant contribution to understanding the mechanisms of regulation of the metabolism of endogenous ethanol and acetaldehyde, their biomedical significance and the biochemistry of the development of alcoholic disease.
Literature
1. Andrianova, L.E. Neutralization of toxic substances in the body / L.E. Andria nova, S.N. Siluyanov a // Biochemistry - 5th ed.; ed. E.S. Severina - M.: GEOTAR-Media, 2009. - S. 619-623.
2. Andronova, L.I. Features of self-stimulation and endogenous ethanol in rats of different sexes / L.I. Andronova, R.V. Kudryavtsev, M.A. Constantinople, A.V. Stanishevskaya // Bull. expert biol. and honey. - 1984. - T. 97, No. 6. - S. 688-690.
3. Burov, Yu.V. N neurochemistry and pharmacology of alcoholism / Yu.V. Burov, N.N. Vedernikova - M.: Medicine, 1985. - 238s.
4. Zavodnik, I.B. Study of the interaction of acetaldehyde with proteins and biologically active compounds / I.B. Zavodnik, N.S. Semukha, I.I. Stepuro, V.Yu. Ostrovsky // Biochemistry of alcoholism; ed. Yu.M. Ostrovsky. - Minsk: Science and technology, 1980.- S. 68.
5. Lakoza, G.N. The level of endogenous ethanol and disorders of testosterone-dependent systems in experimental alcoholism of male white rats / GN. Lakoza, N.V. Tyurina, R.V. Kudryavtsev, N.K. Barkov // I Moscow. scientific and practical. conference of psychiatrists-on-cologists / Issues of pathogenesis, clinics and treatment of alcoholic diseases. - M., 1984.- S. 66-68.
6. Lakoza, G.N. On the Significance of the Central Regulation of Sexual Behavior in Experimental Alcoholism in Male White Rats
/ GN. Lakoza, A.V. Kotov, A.F. Meshcheryakov, N.K. Barkov // Pharma-Col. and toxicol. - 1985. - V. 4, No. 3. - S. 95-98.
7. Lelevich, V.V. The state of the pool of free amino acids in the blood and liver in chronic alcohol intoxication / V.V. Lele-vich, O.V. Artemova // Journal of the Grodno State Medical University. - 2010. - No. 2. - S. 16-19.
8. Ostrovsky, Yu.M. Metabolic concept of the genesis of alcoholism / Yu.M. Ostrovsky // Ethanol and metabolism; ed. Yu.M. Ostrovsky - Minsk: Science and Technology, 1982. - S. 6-41.
9. Ostrovsky, Yu.M. The level of endogenous ethanol and its connection with the voluntary consumption of alcohol by rats / Yu.M. Ostrovsky, M.N. Sadovnik, A.A. Bankovsky, V.P. Obidin // Reports of the Academy of Sciences of the BSSR. - 1983. - T. 27, No. 3. - S. 272-275.
10. Ostrovsky, Yu.M. Ways of ethanol metabolism and their role in the development of alcoholism / Yu.M. Ostrovsky, M.N. Gardener // Results of science and technology. Toxicology. - M.: VINITI, 1984. - Issue. 13. - S. 93-150.
11. Ostrovsky, Yu.M. Biological component in the genesis of alcoholism / Yu.M. Ostrovsky, M.N. Gardener, V.I. Satanovskaya; ed. Yu.M. Ostrovsky - Minsk: Science and Technology, 1986.
12 . Ostrovsky, Yu.M. Metabolic prerequisites and consequences of alcohol consumption / Yu.M. Ostrovsky, V.I. Satanovskaya, S.Yu. Ostrovsky, M.I. Selevich, V.V. Lelevich; ed. Yu.M. Ostrovsky - Minsk: Science and Technology, 1988. - 263 p.
13. Pyzhik, T.N. Pathways for the synthesis of acetaldehyde under conditions of selective inhibition of pyruvate dehydrogenase by oxythiamine
/ T.N. Pyzhik // Journal of Grodno State Medical University. - 2010. - No. 3. - S. 87-88.
14. Solodunov, A.A. Investigation of the effect of alcohols on the binding of ligands by serum albumin / A.A. Solodunov, T.P. Gaiko, A.N. Artsukevich // Biochemistry of alcoholism; ed. Yu.M. Ostrovsky. - Minsk: Science and technology, 1980. - S. 132.
15. Blomstand, R. Observation on the formation of ethanol in the intestinal tract in man / R. Blomstand // Life Sci. - 1971. - Vol. 10. - P. 575-582.
16. Chin, J.H. Increased cholesterol content of erythrocyte and brain membranes in ethanol-tolerant mice / J.H. Chin, L.M. Parsons, D.B. Goldstein // Biochim. Biophys. acta. - 1978. - Vol. 513.-P 358-363.
17 Collins, M.A. Tetraisoquinolines in vivo. Rat brain formation of salsolinol, a product of dopamine and acetaldehyde under certain comditions during ethanol intoxication / M.A. Collins, M.G. Bigdell /
/ Life Sci. - 1975. - Vol. 16.-P 585-602.
18. Higgins, J.J. Biochemistry and pharmacology of ethanol / J.J. Higgins // New Jork-London, 1979. - P 531-539.
19 . Kopczynsk a , T. T he influence of a lcohol dependence on oxidative stress pa ra meters / T. Kopczynsk a , L. Torlinski, M. Ziolkowski // Postepy Hig. Med. Dosw. - 2001. - Vol. 55, No. 1. - P 95-111.
twenty . Lu k a szewicz, A. T he comparison of concentration of endogenous ethanol blood serum in alcoholics and in non-alcoholics at different stages of abstinence / A. Lukaszewicz, T. Markowski, D. Pawlak // Psychiatr. Paul - 1997. - Vol. 31, - P 183-187.
21. Nikolaenko, V.N. Maintenance of homeostasis of endogenous ethanol as a method for the therapy of alcoholism / V.N. Nikolaenko // Bull. Exp. Biol. Med. - 2001. - Vol. 131,
No. 3. - P. 231-233.
2 2 . O strovski, Yu .M. Endogenous etha nol - its metha bolic, behavioral and biomedical significance / Yu.M. Ostrovsky // Alcohol.
1986. - Vol. 3. - P. 239-247.
23. Porasuphatana, S. Inducible nitric oxide syntetase cata lyses ethanol oxidation to alpha-hydroxyethyl ra dica l a nd a cetaldehyde /
With the help of the Excise Legislation, the Government of the Russian Federation limited the production of ethyl alcohol as a motor fuel by setting a high excise rate for ethyl alcohol. The technology for the production of butanol from ethyl alcohol is simple. The production of butanol is exempt from excise tax. The head of Russian Technologies, Sergey Chemezov, is sure that biobutanol from the Tulun hydrolysis plant will be in great demand. Butanol filled three cars that made the Irkutsk-Togliatti rally
1. Ethanol oxidation to obtain Acetaldehyde (acetic aldehyde)
The main industrial method for the production of acetaldehyde CH 3 CHO is the oxidation of ethylene in the presence of aqueous solutions of palladium and copper chlorides. The process is called liquid-phase oxidation of ethylene with oxygen, which is passed through an aqueous solution of PdCl 2 and СuСl 2, then isolated by distillation; the yield is about 98%. In 2003, the global production of Acetaldehyde was about one million tons per year.
2 CH 2 \u003d CH 2 + O 2 → 2 CH 3 CHO
However, this process has a number of disadvantages. This method is characterized by the formation of a number of toxic by-products, such as methyl chloride, ethyl chloride and chloroacetaldehyde, which must be disposed of or subjected to special treatment to prevent environmental pollution. In addition, acetic acid and crotonic aldehyde are formed, which are dissolved in huge amounts of water, which is necessary to isolate the resulting acetaldehyde from a mixture of gaseous products. So, for 1 ton of acetaldehyde produced, there are 8 - 10 m3 of wastewater. In addition, the ethylene used in this process as a feedstock, the production of which is based on the processing of petroleum feedstock, continues to rise in price. Ethylene contract prices in the European market in the fourth quarter of 2004 amounted to 700 euros per tonne, which is 70 euros higher than in the previous quarter, and in September 2004 a peak price level was recorded at 1020 euros per tonne.
Along with this, has not lost its practical value, the process of obtaining Acetaldehyde by catalytic dehydrogenation of ethyl alcohol (ethanol) widely used in the 60s and 70s of the last century. This method has a number of advantages, such as the absence of toxic waste, fairly mild process conditions, and the formation of hydrogen along with acetaldehyde, which can be used in other processes. The raw material is only ethyl alcohol,
Acetaldehyde (acetic aldehyde) is produced from ethyl alcohol by catalytic elimination of hydrogen at ~400°C. Hydrogenation and dehydrogenation are important methods for the catalytic synthesis of various organic substances based on redox-type reactions associated with mobile equilibrium
C2H5OH → CH 3 CHO + H 2

An increase in temperature and a decrease in pressure H 2 contribute to the formation of acetaldehyde, and a decrease in temperature and an increase in pressure H 2 - the formation of ethyl alcohol; such influence of conditions is typical for all reactions hydrogenation and dehydrogenation. Catalysts hydrogenation and dehydrogenation are many metals (Fe, Ni, Co, Pt, Pd, Os, etc.), oxides (Ni O, Co O, Cr 2 O 3, Mo O 2, etc.), as well as sulfides (WS 2, Mo S 2 , Cr n S m).
The dehydrogenation of alcohols is one of the simplest examples of dehydrogenation. When primary or secondary alcohols are passed over the surface of finely divided metals (copper or iron), hydrogen atoms are split off from the alcohol carbon and oxygen of the hydroxyl group (dehydrogenation reaction). In this case, gaseous hydrogen is formed and from the primary alcohol - aldehyde, and from the secondary - ketone. Significant amount acetaldehyde in Russia was produced by dehydrogenation from
For reference:
Acetaldehyde (acetic aldehyde) is the main product of the breakdown of ethanol.
Acetaldehyde (acetic aldehyde) is formed by the oxidation of ethanol, the ethanol oxidation reaction being catalyzed/accelerated mainly by alcohol dehydrogenase. For example, in the human liver, the enzyme (i.e. enzyme) alcohol dehydrogenase oxidizes ethanol to acetaldehyde, which is further oxidized to harmless acetic acid by cetaldehyde dehydrogenase. These two oxidation reactions are associated with the reduction of NAD+ in NADH
During the action on ethyl alcohol of the enzymes indicated in the scheme - alcohol dehydrogenase and aldehyde dehydrogenase - one more substance must participate in the metabolic process. It is a nicotinic acid derivative of NAD. NAD contributes to the inclusion in metabolic processes (burning) of both alcohol and acetaldehyde, while itself being converted into another substance - NADH. In order for the processing of ethyl alcohol not to be interrupted, the liver must convert NADH into NAD.
If both of the processes depicted at the bottom of the ethyl alcohol to acetate chart work effectively, the body will not be threatened by the unpleasant effects of drinking known as a hangover - with a few exceptions that can be dealt with.
If we were drinking pure ethyl alcohol (albeit diluted with water), then the above is all that is required from the liver.  Unfortunately, the drinks we drink in the evening or at dinner are not so pure. Obtained by distillation or fermentation, they contain toxic chemicals. These are the so-called impurities - that is, substances accompanying ethyl alcohol. These include fusel oils, organic acids and even aldehydes. Among these substances are found so toxic that taking them in their pure form would lead to death. To avoid such a danger, it is best to drink alcohol as pure as possible - that is, white wine instead of red, vodka instead of whiskey. To restore good health, impurities that enter the body with alcohol must be included in metabolic processes or destroyed along with alcohol and its by-products.
Unfortunately, the drinks we drink in the evening or at dinner are not so pure. Obtained by distillation or fermentation, they contain toxic chemicals. These are the so-called impurities - that is, substances accompanying ethyl alcohol. These include fusel oils, organic acids and even aldehydes. Among these substances are found so toxic that taking them in their pure form would lead to death. To avoid such a danger, it is best to drink alcohol as pure as possible - that is, white wine instead of red, vodka instead of whiskey. To restore good health, impurities that enter the body with alcohol must be included in metabolic processes or destroyed along with alcohol and its by-products.
In view of the above, very some alternative methods of dealing with a hangover are important. First, the rate at which alcohol enters the body must match its ability to process alcohol into acetaldehyde and then to acetate. This ability is enhanced by eating properly beforehand, and the choice of dishes is indifferent. Fatty foods lubricate the walls of the stomach and duodenum and slow down the absorption of alcohol, proteins help normalize metabolic processes, and carbohydrates adsorb alcohol in the stomach and reduce the intensity of its entry into the bloodstream and muscle tissue.
Secondly, if there are impurities in the drink - say, aldehydes - they should be disposed of. We have two paths before us. It is preferable to collect and absorb acetaldehyde before it enters the blood (the same applies to acetate). Suitable for this charcoal is an excellent adsorbent. No less known to ordinary drinkers are the so-called chelate compounds, which are found, for example, in cabbage. These substances bind harmful elements and remove them from the body. The same is true for vitamin C.
The second less desirable way is the processing of toxins in the body as a result of metabolism. This method is not as efficient: it may be difficult to complete the NAD-NADH-NAD conversion cycle to promote metabolism. Fructose, which is abundant in honey, and oxygen can help here.
Biotechnology allows the production of ethyl alcohol using environmentally friendly technologies from starch-containing crops, as well as sugar-containing agricultural crops, from organic waste and biomass (cellulose) by hydrolysis / conversion by enzymes of microbial origin. At the same time, plant biomass (cellulose), whose role in industrial organic synthesis is constantly increasing as oil and gas reserves are depleted, is a renewable source of organic raw materials and, thanks to a huge annual increase, is able to completely solve human needs for fuel and chemical products. Possibility of use for biological processing of waste and by - products allows to create practically waste - free production . In addition, according to data from the official website of the Danish company "Novozymes", April 14, 2005, www.novozvmes.com, the recent progress in the enzyme industry leads to a significant reduction in the cost of bioethanol production. In the US market, wholesale prices for bioethanol fell by 20% compared to September 2004 and amounted to $44 per barrel at the beginning of April 2005. The bioethanol obtained in this way, in the light of recent trends in the reduction and gradual abandonment of the use of petroleum feedstock, is becoming a very promising intermediate product of organic synthesis and can be used to produce valuable chemical compounds, in particular for the synthesis of acetaldehyde.
2. Obtaining Butanol from Acetaldehyde (acetic aldehyde)
About 1.39 billion liters are produced annually in the US butanol. From acetaldehyde (acetaldehyde) through acetaldol and crotonaldehyde (aldol and croton condensation), which is hydrogenated on copper, copper-chromium or nickel catalysts.
Condensation reactions are usually called various processes of compaction of organic molecules, leading to the formation of more complex compounds as a result of the emergence of new bonds between carbon atoms.
As an example, let us cite the condensation of acetaldehyde under the influence of dilute alkalis (A.P. Borodin, 1863-1873), in which two molecules of aldehyde enter into the reaction; one reacts with a carbonyl group, and the second with a carbon atom in the a-position to a carbonyl group containing a mobile carbon atom, according to the scheme
As a result, a new carbon-carbon bond arises and a substance is formed containing both aldehyde and alcohol groups; it was named aldol(Aldol is an abbreviation for the word aldehyde aldol, i.e. aldehyde alcohol), and the condensation of carbonyl compounds that proceeds along this path and leads to substances such as aldol is calledaldol condensation reaction.
Molecules of various aldehydes, as well as molecules of aldehydes and ketones, can participate in aldol condensation. The latter react at the expense of carbon and hydrogen atoms located in the α-position to their carbonyl group; the carbonyl group itself is less active in these reactions than the carbonyl group of aldehydes.
Under appropriate conditions, the reaction of an aldol condensation of two molecules of an aldehyde, or of a molecule of an aldehyde and a ketone, does not stop at the formation of an aldol; it can go further with the abstraction of water due to mobile hydrogen in the α-position to the carbonyl group and hydroxyl at the β-carbon atom (ie, at the second from the carbonyl group). In this case, as a result of the interaction of two aldehyde molecules, unsaturated (crotonic) aldehyde is formed through the aldol.
From acetaldehyde (acetaldehyde), crotonaldehyde is obtained in this way, from the name of which the condensation of carbonyl molecules, proceeding with the release of water, is calledcroton condensation
Obtaining alcohols from aldehydes and ketones.
We have already seen that during the oxidation of primary and secondary alcohols, substances with a carbonyl group are formed - aldehydes and ketones. Aldehydes and ketones, when exposed to hydrogen at the time of isolation*, are again reduced to alcohols. In this case, the double bond of the carbonyl group is broken and one carbon atom is attached to carbon, and the second to oxygen. As a result, the carbonyl group becomes alcohol.
* Gaseous hydrogen H 2 is inert under normal conditions. Atomic hydrogen, which is released during the reaction of a compound, is very active. This hydrogen is called hydrogen at the time of release.
3. Oxidative dehydrogenation of ethyl alcohol to acetaldehyde on a Sibunit catalyst
To effectively implement the process of dehydrogenation of ethyl alcohol to acetaldehyde with all the above advantages, it is necessary to develop new highly active, selective, and stable catalytic systems. This will make it possible to switch to a more environmentally friendly, and, importantly, independent of petroleum feedstock, method for producing acetaldehyde, which will favorably affect the economy of the process.
An important step in the development of catalytic systems is the search for a catalyst support, which has a great influence on the structure and catalytic properties of the systems. Recently, carbon materials of various types, such as graphite, coke, carbon fibers, diamond, various types of soot and activated carbons, have been used more and more widely in heterogeneous catalytic processes. One of the most promising for use in catalysis is the carbon material sibunit, which is a new class of porous carbon–carbon composite materials. It combines the advantages of both graphite (chemical stability, electrical conductivity) and active carbons (high surface and adsorption capacity). In addition, a very important advantage is its high chemical purity. The proportion of mineral impurities in sibunite is no more than 1%, while the main range of active carbons has an ash content of 5% or more, which has a significant beneficial effect on the selectivity of catalytic systems prepared on the basis of sibunite. This dissertation is devoted to the development of new active and selective catalysts for the synthesis of acetaldehyde by dehydrogenation of ethanol using sibunit carbon material as a carrier, as well as to the determination of the optimal conditions for the process to ensure the efficiency required for industrial applications. The work was carried out at the Department of Technology of Petrochemical Synthesis and Artificial Liquid Fuels. A.N. Bashkirov Moscow State Academy of Fine Chemical Technology. M.V. Lomonosov in accordance with the program "Scientific research of higher education in priority areas of science and technology." Scientific novelty. The process of synthesis of acetaldehyde by dehydrogenation of ethyl alcohol in the presence of copper-containing catalysts based on the carbon-carbon composite material Sibunit has been systematically studied for the first time. It has been shown for the first time that a copper-containing catalyst based on sibunite is most efficient in the reaction of ethanol dehydrogenation, since, unlike oxide supports, side reactions do not occur in the presence of sibunite, which made it possible to increase the selectivity of the studied catalysts in the synthesis of acetaldehyde. The catalytic properties of copper-containing systems based on sibunite were studied depending on the conditions of their pretreatment and the presence of promoting additives. practical value. Efficient copper-containing catalytic systems for the synthesis of acetaldehyde based on the carbon-carbon composite material Sibunit have been developed. Recommendations have been developed for the technological design of the process of synthesis of acetaldehyde by catalytic dehydrogenation of ethanol, which can be used in the design of production facilities. The main content of the dissertation is presented in the following publications: G. Egorova E.V., Trusov A.I., Nugmanov E.R., Antonyuk N., Frantsuzov V.K. The use of carbon materials as carriers for catalysts for the dehydrogenation of low molecular weight alcohols
5. Obtaining butanol. Steam oxidation of diethyl ether.
The formation of esters.
Alcohols react with acids; water is released and the formation esters. The reaction of alcohols with acids is called esterification reaction. With organic carboxylic acids, it proceeds according to the schemeAs we will see later, esters are easily hydrolyzed, i.e., under the action of water, they decompose into the initial alcohol and acid, so the esterification reaction is reversible and reaches a state of chemical equilibrium. We will dwell on this reaction in more detail, as well as on the properties of esters, when we become acquainted with organic acids. Here we only note that the course of the esterification reaction, as shown by N. A. Menshutkin (1877), depends on the structure of alcohol and acid; Primary alcohols are most easily esterified, secondary alcohols are more difficult, and tertiary alcohols are most difficult to esterify.
Alcohols form esters with inorganic (mineral) acids. So, esters of nitric acid are known (nitrate esters)
When alcohols react with polybasic acids, if only one hydroxyl group of the acid reacts, acid esters are formed. For example, dibasic sulfuric acid forms acid esters called alkylsulfuric acids
Alkyl sulfuric acids are formed as intermediate products in the reactions of hydration of unsaturated hydrocarbons and dehydration of alcohols under the action of sulfuric acid.
Under the action of water-removing agents, for example, when heated with concentrated sulfuric acid, alcohols lose a water molecule; moreover, depending on the reaction temperature and the quantitative ratios of alcohol and sulfuric acid, two cases of dehydration are possible. In one of them, the withdrawal of water occurs intramolecular, i.e. due to one molecule of alcohol, with the formation of ethylene hydrocarbon
In another case, with an excess of alcohol, dehydration proceeds intermolecular, i.e., by isolating a water molecule at the expense of the hydroxyl groups of two alcohol molecules; this creates the so-called ethers:
The role of sulfuric acid in the intramolecular dehydration of alcohols, leading to the production of ethylene hydrocarbons, has already been considered,
Diethyl (ethyl) ether. It is of great practical importance; it is usually called simply ether. It is obtained mainly by the dehydration of ethyl alcohol by the action of concentrated sulfuric acid. By this method, diethyl ether was obtained for the first time as early as 1540 by V. Kordus; for a long time diethyl ether was misnamed sulfur ether, since it was supposed to contain sulfur. Currently, diethyl ether is obtained in the same way by passing ethyl alcohol vapor over alumina
Al 2 O 3 , heated to 240-260°C.Diethyl ether is a colorless, volatile liquid with a characteristic odor. Pace. bale 35.6°С, freezing rate - 117.6°С; cp=0.714, i.e. ether is lighter than water. If it is shaken with water, then when standing, the ether “exfoliates” and floats to the surface of the water, forming the upper layer. However, a certain amount of ether dissolves in water (6.5 hours in 100 hours of water at 20°C). In turn, at the same temperature, 1.25 parts of water dissolve in 100 parts of ether. Ether mixes very well with alcohol.
It is important to keep in mind that ether must be handled with care: it is very flammable, and its vapors with air form explosive - explosive mixtures. In addition, during long-term storage, especially in the light, the ether is oxidized by atmospheric oxygen and the so-called peroxide compounds*; the latter from heating can decompose with an explosion. Such explosions are possible during the distillation of ether that has stood for a long time.
Hydroiodic acid decomposes ethers; the result is a haloalkyl (iodo derivative) and an alcohol
Wurtz synthesis consists in obtaining hydrocarbons from halogen derivatives by the action of metallic sodium on them. The reaction proceeds according to the scheme
For example,
From butane, isomerization can be used to obtain isobutane, which can serve as a raw material for isobutylene production by isobutane dehydrogenation. Subsequent esterification of isobutylene with ethyl alcohol produces an oxygen-containing additive to gasoline - environmentally friendly ethyl tert-butyl ether (ETBE), having an octane number of 112 points (Research method).
Physical properties of normal chain primary haloalkyls
| Chloride | bromide | iodide | |||||
| title | structure | boiling point, °C | d 4 20 | boiling point, °C | d4 20 | boiling point, °C | d 4 20 |
| Methyl | CH 3 - | -23,7 | 0,992* | +4,5 | 1,732** | + 42,5 | 2,279 |
| Ethyl | CH 3 - CH 2 - | + 13,1 | 0,926*** | +38,4 | 1,461 | +72,3 | 1,936 |
| Propil | CH 3 - CH 2 - CH 2 - | + 46,6 | 0,892 | +71,0 | 1,351 | + 102,5 | 1,749 |
| Butyl | CH3 - (CH 2) 2 - CH 2 - | +78,5 | 0,887 | + 101,6 | 1,276 | + 130,4 | 1,615 |
| Amyl | CH 3 - (CH 2) 2 - CH 2 - | + 108,4 | 0,878 | + 127,9 | 1,218 | +154,2 | 1,510 |
| Hexyl | CH 3 - (CH 2) 4 - CH 2 - | + 132,9 | 0,876 | + 153,2 | 1,176 | + 177,0 | 1,439 |
* At boiling point.
***d40
In the presence of catalysts at high temperatures, hydrogen is split off (dehydrogenation reaction) from molecules of saturated hydrocarbons with the formation of double bonds. Thus, when butane is passed over a catalyst containing heavy metal oxides (for example,
Cr2O3 ), at temperatures of 400 - 600º a mixture of butylenes is formedAccession of water (hydration reaction). Under normal conditions, ethylene hydrocarbons do not react with water, but when heated in the presence of catalysts (zinc chloride, sulfuric acid), water elements (hydrogen and hydroxyl) are added to carbon atoms at the double bond site to form alcohols
With ethylene homologues, the reaction proceeds according to Morkovnikov's rule: the hydrogen of water is added to the carbon at which there are more carbon atoms, and the hydroxyl is added to the carbon at which there are fewer or no hydrogen atoms.
This method gives a special opportunity to use as a raw material for the production of butane, butyl alcohol ... ethyl alcohol
Of great interest is the production of isobutylene from ethyl alcohol. First, normal butane (n-butane) is obtained from ethanol as described above. Isobutane is obtained from n-butane by catalyzed isomerization. From isobutane, isobutylene is obtained - as a raw material for the production of an anti-knock additive in gasoline - ethyl tert-butyl ether ETBE. This method gives a special opportunity to use ethyl alcohol as a raw material for isobutylene production. Thus, only ethyl alcohol without isobutylene is used to obtain ETBE.
1. Lebedev N.N. Chemistry and technology of basic organic and petrochemical synthesis. 4th ed. Moscow: Chemistry, 1988. 592 p.
2. Timofeev B.C., Serafimov L.A. Principles of basic organic and petrochemical synthesis technology. 2nd ed. M.: Higher school, 2003. 536 p.
3. Steppich W., Sartorius R. Process for the manufacture of acetaldehyde. U.S. Patent 4237073, Dec. 2, 1980 (US CI. 568/401).
4. Khcheyan X.E., Lange C.A., Ioffe A.E., Avrekh G.L. Acetaldehyde production. M.: TsNIITE Neftekhim, 1979. 40 p.
5. Kuznetsov B.N. Plant biomass is an alternative raw material for low-tonnage organic synthesis Ros. chem. journal (Zh. Ros. chem. ob-va named after D.I. Mendeleev). 2003, vol. XLVII, 6. 3.
6. Kukharenko A.A., Vinarov A.Yu., Sidorenko T.E., Boyarinov A.I. Intensification of the microbiological process of obtaining ethanol from starch- and cellulose-containing raw materials. M., 1999. 90 p.
7. Plaksin G.V. Porous carbon materials such as sibunit. Chemistry for sustainable development. 2001, No. 9. 609-620.
8. Semikolenov V.A. Modern approaches to the preparation of catalysts "Pallady!! on the corner." advances in chemistry. 1992, v. 61, no. 2. 320-331.
9. Berg World fuel ethanol analysis and outlook "P.O. Licht" agency. http://www.distill.com/World-Fuel-Ethanol-A&O-2004.html lO.Berg C. World ethanol production and trade to 2000 and beyond "P.O. Licht" agency. http://www.distill.eom/ber.g/ P.Volkov B.V., Fadeev A.G., Khotimsky B.C., Buzin O.I., Tsodikov M.V., Yandieva F.A., Moiseev I.I. . Environmentally friendly biomass fuel Ros. chem. journal (Zh. Ros. chem. ob-va named after D.I. Mendeleev). 2003, vol. XLVII, 6. P 71-82. 188
10. Kadieva A.T. Development of intensive ethanol technology based on the targeted use of multienzyme systems and new races of alcoholic yeast: Dis....cand. those. Sciences. Moscow, 2003.
11. Lukerchenko V.N. Non-starchy grain carbohydrates and their importance for alcohol production Food industry. 2000, No. 1. 62-63.
12. Rimareva L.V. Technology for obtaining promising enzyme preparations and features of their use in the alcohol industry Modern and progressive technologies and equipment in the alcohol and alcoholic beverage industry 2nd International Scientific and Practical Conference. Moscow: Pishchepromizdat, 2000. 48-63. 1 Z. Kalinina O.A. Development of a resource-saving technology for producing ethanol from rye grain: Dis....cand. those. Sciences. Moscow, 2002.
13. Lichtenberg L.A. Production of alcohol from grain Food industry. -2000, 7 C 52-54.
14. Alcohol technology. Ed. V.L. Yarovenko, M.: Kolos, 1999. 464 p.
15. Rimareva L.V., Overchenko M.B., Trifonova V.V., Ignatova N.I. Osmophilic yeast for fermentation of highly concentrated wort Production of alcohol and liqueurs. 2001, No. 1. 21-23.
16. V. A. Bondarenko, V. L. Kasperovich, V. A. Butsko, and E. Sh. A method for preparing grain starch-containing raw materials for alcoholic fermentation. RF patent No. 2145354, Appl. 11/24/1998, publ. February 10, 2000 (IPC C12 R7/06).
17. Sviridov B.D., Lebedev Yu.A., Zaripov R.Kh., Kutepov A.M., Antonyuk A.V. Method for the production of ethyl alcohol from grain raw materials. RF patent 2165456, Appl. 05/19/2000, publ. 04/20/2001 (IPC C12 R7/06).
18. Timoshkina N.E., Kretechnikova A.N., Imyashenko N.G., Shanenko E.F., Gernet M.V., Kirdyashkin V.V. Yeast processing method. RF patent No. 2163636, Appl. 03/30/2000, publ. 02/27/2001 (IPC С12 N1/16).
19. Zhurba O.S. Development of a new ethanol technology based on intensive methods of processing wheat grain: Dis....cand. those. Sciences. Moscow, 2004. 189
20. Vasil'eva N.Ya., Rimareva L.V. Fermentation of starch-containing raw materials by anaerobic bacteria of the genus Zymomonas Production of alcohol and alcoholic beverages. 2001, No. 1. 18-20. 25. Fedorov A.D., Kesel B.A., Dyakonsky P.I., Naumova R.P., Zaripova K., Veseliev D.A. Method for producing ethyl alcohol. RF patent 2138555, Appl. 12/05/1997, publ. 09/27/1999 (IPC C12 R7 / 06).
21. Ledenev V.P. Status and tasks for improving the technology of production of alcohol from grain at the plants of the Russian Federation. Enzymes in the food industry. Conference abstracts. M., 1999. 22-27.
22. Mulder M.H.V., Smolders A., Bargeman D. Membraan filtratie bij de produktie van ethanol PT Procestechniek. 1981, 36, no. 12. P. 604-607.
23. Mori Y., Inaba T. Ethanol production firom starch in a pervaporation membrane bioreactor using Clostridium thermohydrosulfuricum Biotechnology and Bioengineering. 1990, v. 36, 8. P.849-853.
24. Gamil A. Conversion of sugar-beet particles to ethanol by the bacterium Zymomonas mobilis in solid-state fermentation Biotechnology Letters. 1992, 14, 6 P 499-504.
25. Saxena A, Garg S.K., Verma J. Simultaneous saccharificatiom and fermentation of waste newspaper to ethanol Bioresour. technology. 1992, 42, 1. P. 13-15.
26. Grohmann K., Baldwon E. A., Buslig B. S. Production of ethanol from enzymatically hydrolyzed orange peel by the yeast Saccharomyces cerevisiae // Applied Biochemistry and Biotechnology A. 1994, 45-46. P. 315-327.
27. Mangueva 3.M. Patterns of growth of cell culture Saccharomyces cerevisiae (vini)Y 2217 in the biosynthesis of ethanol from apricot must: Abstract of the thesis. cand. chem. Sciences. Makhachkala, 2004. 190
28. Tolan J.S. logens process for producing ethanol from cellulosic biomass Clean Techn. Environ. policy. 2002, No. 3. p. 339-345.
29. Loktev S.M., Korneeva G.A., Mosesov A.Sh., Kuimova M.E. Obtaining products of basic organic synthesis from the simplest carbon compounds. M.: VNTICenter, 1985. 132 p. Zb.Putov N.M. Production of acetic acid and acetic anhydride abroad. M.: Goshimizdat, 1948. 56 p.
30. Kalfus M.K., Khasanov A.S. Industrial liquid-phase oxidation of acetaldehyde to acetic acid. Alma-Ata, 1958. 16 p.
31. Chashchin A.M., Glukhareva M.I. Production of acetate solvents in the wood chemical industry. M.: Les. industry, 1984. 240 p.
32. Chernyak B.I., Savitsky Yu.V., Ernovsky N.P., Vvasilenko O.R., Kibin F.S., Vine V.V., Kravtsov N.I. The method of obtaining ethyl acetate. RU 2035450 C1, application. 04/01/1991, pub. May 20, 1995 (IPC C07 C69/14).
33. Wittcoff N.A. Acetaldehyde: a chemical whose fortunes have changed Journal of chemical education. 1983, 60, no. 12. P. 1044-1047.
34. Yalter Yu.A., Brodsky M.S., Feldman B.M. Method for obtaining glyoxal, A.S. No. 549457, application. 08/08/1974, pub. 03/05/1977 (IPC C07 C47 / 127). 42, Lehmann R.L., Lintner J. Manufacture of glyoxal and polyglyoxal. US Patent 2599355, June 3, 1952 (US CI. 568/458).
35. Chemical encyclopedia: In 5 volumes: v. 3. Ed. Col.: Knunyants I.L. (ch.; ed.) and others. Moscow: Great Russian Encyclopedia, 1992. 639 p. 44. Eek L. Process for preparation of pentaerythritol. U.S. Patent 5741956, Apr. 21, 1998 (US CI. 568/853).
36. Vebel H.I., Moll K.K., Muchlstacdt M. Preparation of 3-methylpyridine Chemishe Technik. 1970.22, No. 12. P. 745-752.
37. Dinkel P. Method for obtaining 3-picoline. SU 1095876 A, Appl. 05/22/1981, pub. May 30, 1984 (IPC C07 D213/10). 191
38. Ladd E.S. Production of ketoesters. U.S. Patent 2533944, Dec. 12, 1950 (US CI. 560/238).
39. Vinogradov M.G., Nikishin G.I., Stepanova G.A., Markevich B.C., Markevich S.M., Baybursky V.L. Method for obtaining y-acetopropyl acetate. A.S. 504753, App. 03/19/1973, pub. 02/28/1976 (IPC C07 C69 / 14).
40. Anikeev I.K., Madiyarova Kh.Sh., Nefyodov O.M., Nikishin G.I., Dolgii I.E., Vinogradov M.G. A method for producing acetopropyl alcohol acetate. A.S. 614091, App. 06/09/1975, pub. 07/05/1978 (IPC C07 C69 / 14).
41. Tustin G.C., Zoeller J.R., Depew L.S. Process of preparation of vinyl acetate. U.S. Patent 5719315, Feb. 17, 1998 (US CI. 560/238). 52. Wang C.-G. Preparation of vinyl acetate. GB 2013184 A, Dec. 29, 1978 (IPC C07 C69/15).
42. Eller K., Fiege B., Henne A., Kneuper H.-J. Preparation of Nethyldiisopropylamine. U.S. Patent 6111141, Aug. 29, 2000 (US CI. 564/473).
43. Wakasugi T., Miyakawa T., Suzuki F. Process for the manufacture of monochloroacetaldehyde trimer and chloral. US Patent 5414139, May 9, 1995 (US CI. 568/466).
44. Dhingra Y.R. Alpha chlorination of acid chlorides. US Patent 3751461, Aug. 7, 1973 (US CI. 562/864).
45. Ebmeyer F., Metzenthin T., Siegemund G. Process for the preparation of trichloroacetyl chloride. U.S. Patent 5659078, Aug. 19, 1997 (US CI. 562/864). 57. Abe T., Gotoh T., Uchiyama T., Hoguchi H., Shima Y., Ikemoto K. Process for preparing lactate. US Patent 5824818, Oct. 20, 1998 (US CI. 560/179).
46. Young D.C. Oxidation of olefins. US Patent 3850990, Nov. 26, 1974 (US CI. 568/449).
47. Copelin H.B. Process for producing acetaldehyde from ethylene. US Patent 3531531, Sep. 29, 1970 (US CI. 568/484). 192
48. Robinson D.V. Method for obtaining carbonyl compounds. A.S. 444359, App. 12/07/1972, pub. 09/25/1974 (IPC C07 C47 / 07).
49. Nishimura Y., Yamada M., Arikawa Y., Kamiguchi T., Kuwahara T., Tanimoto H. Process for producing acetaldehyde. US Patent 4521631, Jun. 4, 1985 (US CI. 568/478).
50. Sokolsky D.V., Nurgozhaeva Sh.Kh., Shekhovtsev V.V. Method for the production of acetaldehyde. A.S. 171860, App. 06/24/1964, pub. 06/22/1965 (С07 С47/06).
51. Flid R.M., Tyomkin O.N., Streley M.M. Method for the production of acetaldehyde. A.S. 175943, App. 09/26/1962, pub. 10/26/1965 (IPC C07 C47/06).
52. Agladze R.I., Gegechkori V.L. Method for the production of acetaldehyde. A.S. 177870, App. 05/13/1963, pub. 01/08/1966 (IPC C07 C47 / 06).
53. Petrushova N.V., Kirillov I.P., Peskov B.P. A method for the joint production of acetaldehyde and acetic acid. A.S. J b 387963, Appl. N 20.09.1971, pub. 06/22/1973 (IPC C07 C47 / 06).
54. Gorin Yu.A., Troitsky A.N., Makashina A.N., Gorn I.K., Derevyagina N.L., Mamontov B.V. et al. Method for obtaining acetic and cratonic aldehydes by vapor-phase hydration of acetylene. A.S. 138607, App. 08/22/1960, pub. 1961 (IPC C07 C47/06).
55. Kirshenbaum I., Amir E.M., InchaHk J. Oxidation of alcohols. U.S. Patent 3080426, Mar. 5, 1963 (US CI. 568/487).
56. Sanderson J.R., Marquis E.T. Oxidation of primary alcohols to aldehydes using transition metal phthalocynines as catalyst. U.S. Patent 5132465, Jul. 21, 1992 (US CI. 568/485). 70, Nagiyev T.M., Zulfugarova C.3., Iskenderov P.A. Method for the production of acetaldehyde. A.S. 891623, App. 04/09/1980, pub. 12/23/1981 (IPC C07 C47/06). 193
57. Volkova A.N., Smirnov V.M., Koltsov S.I. Ivanova L.V., Yakovlev V.I. Method for preparing a silver catalyst for the oxidation of ethyl alcohol. A.S. 753459, App. 04/12/1978, pub. 08/07/1980 (IPC C07 C47 / 07).
58. Hudlicky M. Oxidation in organic chemistry ACS Monograph. 1990, 186, pp. 114-126.
59. Kannan S., Sivasanker S. Catalytic behavior of vanadium containing molecular sieves for selective oxidation of ethanol 12- Proc. Int. Zeolite Conference. 1998 (Pub 1999), 2. P. 877 884.
60. Kozminykh O.K., Makarevich N.A., Ketov A.N., Kostin L.P., Burnyshev V.C. Method for the production of acetaldehyde. A.S. 352874, App. 05/22/1970, pub. 09/29/1972 (IPC C07 C47 / 06).
61. Aleksandrov Yu.A., Tarunin B.I., Pereplyotchikov M.L., Pereplyotchikova V.N., Klimova M.N. Method for the production of acetaldehyde. A.S. 891624, App. 11/16/1979, pub. 12/23/1081 (IPC C07 C47/06).
62. Pretzer W.R., Kobylinski T.P, Bozik J.E. Process for the selective preparation of acetaldehyde from methanol and synthesis gas. US Patent 4151208, Apr. 24, 1979 (US CI. 568/487).
63. Pretzer W.R., Kobylinski T.P., Bozik J.E. Process for producing acetaldehyde. U.S. Patent 4239704, Dec. 16, 1980 (US. CI. 568/487).
64. Keim K.-H., Kroff J. A process for the production of acetaldehyde and ethanol. GB 2088870 A, 4 Dec. 1981 (IGJ C07 C47/06).
65. Pretzer W.R., Kobylinski T.P., Bozik J.E. Process for producing acetaldehyde. U.S. Patent 4239705, Dec. 16, 1980 (US. CI. 568/487).
66 Larkin Jr. T.N., Steinmetz G.R. Process for the preparation of acetaldehyde. US Patent 4389532, Jun. 21, 1983 (US CI. 568/487).
67. Rizkalla N. Preparation of acetaldehyde. US Patent 4628121, Dec. 9, 1986 (US CI. 568/487).
68. Wegman R.W., Miller D.S. Synthesis of aldehydes from alcohols. US Patent 4594463, Jun. 10, 1986 (US CI. 568/487).
69. Walker W.E. Process for the selective hydroformylation of methanol to acetaldehyde. US Patent 4337365, Jun. 29, 1982 (US CI. 568/487).
70. Porcelli R.V. Preparation of acetaldehyde. US Patent 4302611, Nov. 24, 1981 (US CI. 568/484).
71. Hajime Y., Yoshikazu S. Method for manufacturing acetaldehyde. JP 256,249 Sep. 19, 2000 (YYC C07 C45/54).
72. Isogai N., Hosokawa M., Okawa T., Wakui N., Watanabe T. Process for producing acetaldehyde. US Patent 4408080, Oct. 4, 1983 (US CI. 568/484).
73. Nakamura S., Tamura M. Process for producing acetaldehyde. US Patent 4351964, Sep. 28, 1982 (US CI. 568/484). 95. Moy D. Process for the preparation of acetaldehyde. US Patent 4356328, Oct. 26, 1982 (US CI. 568/484). 195
74. Tustin G.S., Depew L.S., Collins N.A., Method for producing acetaldehyde from acetic acid. US Patent 6121498, Sep. 19, 2000 (US CI. 568/420).
75. Rachmady W., Vannice M.A. Acetic acid reduction to acetaldehyde over iron catalysts. I. Kinetic behavior Journal of catalysis. 2002, 208, No. 1. P. 158-169.
76. Rachmady W., Vannice M.A. Acetic acid reduction to acetaldehyde over iron catalysts. II. Characterization by Mossbauer spectroscopy, DRIFTS, TPD and, TPR Journal of catalysis. 2002, 208, No. 1. P. 170-179.
77. Fenton D.M. Conversion of chloroformates to an aldehyde. U.S. Patent 3720718, Mar. 13, 1973 (US CI. 5.68/484).
78. Roscher G., Schmit K., Schmit T., Schmit H. Process for the manufacture of acetaldehyde from vinil acetate. U.S. Patent 3647882, Mar. 7, 1972 (US CI. 568/484).
79. Shostakovsky M.F., Azerbaev I.N., Yakubov R.D., Atavin A.S., Petrov L.P., Shvetsov N.V. etc. Method for producing acetaldehyde. A.S. 222363, App. 12/27/1965, pub. 07/22/1962 (IPC C07 C47 / 06).
80. Parker R.T. Preparation of aldehydes by steam oxidation of ethers. US Patent 2477312, Jul. 26, 1949 (US CI. 568/485).
81. Fenton D.M. Decomposition of carbonates to form aldehydes. U.S. Patent 3721714, Mar. 20, 1973 (US CI. 568/449).
82. Neely S.D. Conversion of ethyl alcohol to acetaldehyde. US Patent 3106581, Oct. 8.1963 (US CI. 568/471). 105. MacLean A.F. Process for catalytic dehydrogenation of alcohols to carbonyl compounds. U.S. Patent 2634295, Apr. 7, 1953 (US CI. 568/406).
83. Marcinkowsky A.E., Henry J.P. Catalytic dehydrogenation of ethanol for the production of acetaldehyde and acetic acid. US Patent 4220803, Sep. 2, 1980 (US CI. 562/538).
84. Allahverdova H.X. Gas-phase transformations of ethanol into oxygen-containing products on complex oxide catalysts. Abstract Dis. doctor of chemical sciences. Baku, 1993. 196
85. Backhaus A.A., Arentz F.B. Process of making aldehydes. US Patent 1388841, Aug. 30, 1921 (US CI. 568/487).
86. Williams C.S. Process for making acetic aldehyde. US Patent 1555539, Sep. 29, 1925 (US CI. 568/487).
87. Raich B.A., Foley Henry C Ethanol dehydrogenation with a palladium membrane reactor: an alternative to Wacker chemistry Ind. Eng. Chem. Res. 1998, 3 7 P 3888-3895.
88. Matsumura Y., Hashimoto K., Yoshida S. Selective dehydrogenation of ethanol over highly dehydrated silica Journal of catalysis. 1989, 117. P. 135-143.
89. Carrasco-Marin F., Mueden A., Moreno-Castilla C Surface-treated activated carbons as catalysts for the dehydration and dehydrogenation reactions of ethanol Journal of physical chemistry B. 1998, 102. P. 9239-9244. 114. Bo-Quing X., Tian-Xi C, Song L. Selective dehydrogenation of ethanol to acetaldehyde over Na ZSM-5 calcined at high temperature Reaction kinetics and catalysis letters. 1993, 49, No. 1. P. 223-228.
90. Iwasa N., Takezawa N. Reforming of ethanol dehydrogenation to ethyl acetate and steam reforming to acetic acid over copper-based catalysts Bulletin of the chemical society of Japan. 1991, 64. P. 2619-2623.
91. Chen D.A., Freind C M Selective and nonselective dehydrogenation in primary alcohols: reactions of ethanol and 1-propanol on Co-covered Mo (110) Langmuir.-1998, 14.-P. 1451-1457.
92. Idriss H., Seebauer E.G. Reactions of ethanol over metal oxides Journal of molecular catalysis A. 2000, 152. P. 201-212. 118. Kim K.S., Barteau M.A., Farneth W.E. Adsoftion and decomposition of aliphatic alcohols on Ti02 Langmuir. 1988.4, No. 3. P. 533-543. 197
93. Cong Y., Masel R.I., van Spaendonk V. Low temperature C-C bond scission during ethanol decomposition on on Pt (331) Surface science. 1997, 385, No. 2-3.-P. 246-258.
94. Inui K., Kurabayashi T., Sato S. Direct synthesis of ethyl acetate carried out under pressure Journal of catalysis. 2002, 212. P. 207-215.
95. Iwasa N., Yamamoto O., Tamura R., Nishikybo M., Takezawa N. Difference in reactivity of acetaldehyde intermediates in the dehydrogenation of ethanol over supported Pd catalysts Catalysis letters. 1999, 62. P. 179-184.
96. Matsumura Y., Hashimoto K., Yoshida S. Selective dehydrogenation of ethanol to acetaldehyde over silicalite-1 Journal of catalysis. 1990, 122. P. 352-361.
97. Chung M-J., Moon D-J., Kim H-S., Park K-Y., Ihm S-K. Higher oxygenate formation from ethanol on Cu/ZnO catalysts: Synergism and reaction mechanism Journal of molecular catalysis A. 1996, 113. P. 507-515.
98. Sexton B.A. Surface vibrations of adsorbed intermediates in the reactions of alcohols with Cu(lOO) Surface science. 1979, 82. P. 299-318.
99. Elliot D.J., Penella F. The formation of ketones in the presence of carbon monoxide over CuO/ZnO/AbOa// Journal of catalysis. 1989, 119, no. 2. P. 359ПТЗbSheldon P.A. Chemical products based on synthesis gas. Per. from English. ed. Lokteva SM. Moscow: Chemistry, 1987. 248 p.
100. Matsumura Y., Hashimoto K., Watanabe S., Yoshida S. Dehydrogenation of ethanol over ZSM-5 type zeolites Chemistry letters. 1981, No. 1. P. 121-122. 129. J.M., Joshi H.K. Acetaldehyde by dehydrogenation of ethyl alcohol Industrial and engineering chemistry. -1951, August. P 1805-1811.
101. Process for the dehydrogenation of alcohols. GB 825602, 12/16/1959 (IPC C07 C45/00D). 198
102. Young CO. Process for making acetaldehyde and a catalyst therefor. US Patent 1977750, Oct. 23, 1934 (US CI. 568/487).
103. Borisov A.M., Lapshov A.I., Malyutin N.R., Karasev V.N., Gaivoronsky V.I., Nikitin Yu.S., Bashilov L.S. Method for the production of acetaldehyde. A.S. 618368, App. 07/01/1974, pub. 08/05/1978 (IPC C07 C47 / 06). 134. Ty Y-J., Chen Y-W. Effects of alkaline-earth oxide additives on silicasupported copper catalysts in ethanol dehydrogenation Ind. Eng. Chem. Res. 1998, 3 7 P 2618-2622.
104. Kanuon N., Astier M.P., Pajonk G.M. Selective dehydrogenation of ethanol over Cu catalysts containing Zr or V and Zr React. Kinet. catal. Lett. 1991, 44, 1 P 51-56.
105. Kawamoto K., Nashimura Y. The catalytic reaction of alcohols with reduced copper Bulletin of the chemical society of Japan. 1971, 44. P. 819-825.
106. Komarewski V.I. Dehydrogenation of alcohols. U.S. Patent 2884460, Apr. 28, 1959 (US CI. 568/485).
107. Sultanov A.S., Makhkamov X.M., Sapozhnikova E.A., Yanova A.E., Lapinov A.I., Borisov A.M. Method for the production of acetaldehyde. A.S. 433782, App. 02/24/1971, pub. 02/25/1976 (IPC C07 C47 / 06).
108. Teshchenko A.D., Kursevich O.V., Klevchenya D.I., Andreevsky D.N., Sachek A.I., Basiev I.M., Andreev V.A. Ethanol dehydrogenation catalyst. A.S No. 1109189, application. 02/22/1981, pub. 08/23/1984. (IPC C07 C47/06).
109. Duncanson L.A., Charman N.V., Coffey R.S. Dehydrogenation of alcohols. GB 1061045, 03/08/1967 (IPC C07 C45/00D).
110. Setterfield C. Practical course of heterogeneous catalysis: TRANS. from English. M.: Mir, 1984.-520 s,
111. Savelyev A.P., Dyment O.N., Borisov A.M., Kantor A.Ya., Kaluzhsky A.A., Oleinikova N.S.
112. Areshidze Kh.I., Chivadze G.O., Iosiliani D.K. Method for the production of aldehydes and ketones. A.S. No. 400570, application. 07/12/1971, pub. 10/01/1973 (IPC C07 C47 / 06). 144. Veryovkin P.F., Malyutin N.R., Smirnov A.I. Method for the production of acetaldehyde. A.S. No. 191519, application. 12/17/1965, pub. 01/26/1967 (IPC C07 C47 / 06).
113. Deng J., Cao Y., Liu B. Catalytic dehydrogenation of ethanol in Pd-M/y-AOs composite membrane reactors Applied Catalysis. 1997, 154, no. 1-2. P. 129138.
114. Schmitt J.L., Walker P.L., Castellion G.A. Carbon particles with controlled density. U.S. Patent 4,029,600 Jun. 14, 1977 (US CI. 502/418).
115. Yermakov Yu.L, Surovikin V.F., Plaksin G.V., Semikolenov V.A., Likholobov V.A., Chuvalin L.V., Bogdanov S.V. New carbon material as support for catalysts Reaction kinetics and catalysis letters. 1987, 33, no. 2. P. 435-440.
116. Surovikin V.F., Plaxin G.V., Semikolenov V.A., Likholobov V.A., Tiunova I.J. Porous carbonaceous material. U.S. Patent 4978649, Dec. 18, 1990 (US CI. 502/416).
117. Surovikin V.F., Fenelonov V.B., Plaksin G.V., Semikolenov B.A., Okkel L.G. Patterns of Formation of the Porous Structure of Composites Based on Pyrolytic and Technical Carbon Solid Fuel Chemistry. 1995, No. 3. 62-68.
118. Gavrilov V.Yu., Fenelonov V.B., Chuvilin A.L., Plaksin G.V., Surovikin V.F., Ermakov Yu.I., Semikolenov V.A. Study of the morphology and porous structure of composite carbon-carbon materials Solid fuel chemistry. 1990, No. 2. 125-129.
119. Plaksin G.V., Surovikin V.F., Fenelonov V.B., Semikolenov V.A., Okkel L.G. Formation of the texture of a new carbon support for catalysts Kinetics and catalysis. 1993.34, No. 6. 1079-1083. 200
120. Fenelonov V.B. Introduction
121. Semikolenov V.A. Design of highly dispersed carbon-supported palladium catalysts Journal of Applied Chemistry. 1997, 70, No. 5.-S. 785-796.
122. Startsev A.N., Shkuropat A., Zaikovsky V.I., Moroz E.M., Ermakov Yu.I., Plaksin G.V., Tsekhanovich M.S., Surovkin V.F. Structure and catalytic properties of carbon-supported sulfide hydrodesulfurization catalysts Kinetics and catalysis. 1988, v. 29, no. 2. 398-405.
123. Korolkov V.V., Doronin V.P., Startsev A.N., Klimov O.V., Turekhanova R.N., Duplyakin V.K. Hydrodemetallization of Vanadylporphyrins on Mo and Ni-Mo Sulfide Catalysts Supported on Sibunit Kinetics and Catalysis. 1994.35, No. 1. 96-99.
124. Ryashentseva M.A., Avaev V.I. Hydrogenation of ethyl acetate on supported rhenium catalysts Proceedings of the Academy of Sciences. Chemical series. 1999, No. 5.-S. 1006-1008.
125. Ryashentseva M.A. Properties of supported rhenium catalysts in the dehydrogenation of cyclohexane Izvestiya Akademii Nauk. Chemical series. 1996, No. 8.-S. 2119-2121.
126. Ryashentseva M.A. Selective dehydrogenation of isopropyl alcohol on low molecular weight supported bimetallic rhenium-containing catalysts Izvestiya Akademii Nauk. Chemical series. 1998, No. 11. 2381-2383. 201
127. SV Zemskov, L.L. Gornostaev, V.N. Mitkin, Yu.I. Ermakov, A.S. Lisitsyn, V.A. Likholobov, I.A. Kedrinsky, V.P. .V., Surovikin V.F. Fluoric carbon and method for its production. RF patent No. 2054375, Appl. 05/15/1987, publ. 02/20/1996 (IPC C01 B31/00).
128. Kovalenko G.A., Semikolenov V.A., Kuznetsova E.V., Plaksin G.V., Rudina N.A. Carbon materials as adsorbents for biologically active substances and bacterial cells Colloid journal. 1999, 61, No. 6. 787-795.
129. Yakerson V.I., Golosman E.Z. Catalysts and cements. M.: Chemistry, 1992. -256 p.
130. Nissenbaum V.D. Formation, surface and catalytic properties of contacts based on calcium aluminates: Dis....cand. chem. Sciences. Moscow, 1989.
131. Rodriguez-Reinoso F. The role of carbon materials in heterogeneous catalysis Carbon. 1998, 36, no. 3. P. 159-175. 166. P.A. Lydia, B.A. Milk, L.L. Andreeva Chemical Properties of Inorganic Compounds Ed. R.A. Lidina. Moscow: Chemistry, 1996. 480 p.
132. Surface analysis by Auger and X-ray photoelectron spectroscopy: per. from English. ed. D. Briggs and M.P. Seeha. M.: Mir, 1987.-600 p. 202
Andrei Kamensky, head of the Department of Physiology at the Faculty of Biology, Moscow State University, says that he was always surprised how such a simple molecule as ethanol, which astronauts discovered even in space, triggers a huge mass of complex reactions in the body. This action can be compared to the effect of a falling domino in a design that has been posted for several months. But there is an unpleasant difference - the body can no longer be reassembled.
Alcohol is loved for the fact that you can become cheerful, impulsive and daring. Scientists do not deny that a small stimulation of neurons enhances mental activity, including thinking. Alcohol can also enhance analytical skills. Too shy - help in communication. Hunters - keep an eye out. Alcohol even fights with cholesterol plaques in the vessels. But talking about the few virtues of alcohol makes sense, if you know the measure. And what is the measure is also a question.
Scientists say that the pleasure of alcohol is unnatural. The researchers are impartial: “alcohol seems to cut off the path to the pleasure center in our brain.” “Natural” enjoyment, such as music, a job well done, or sex, has a longer path through a complex system of checks and balances that evaluates information and doses rewards. And alcohol just rudely breaks into this center. It turns out like in a win-win lottery - a big win for nothing. It turns out that a person himself, with the help of biochemical reactions unknown to him, achieves a certain effect, similar to electrical stimulation of the brain on the pleasure center.
Once ingested, alcohol is rapidly absorbed into the bloodstream, first in the stomach, but to a greater extent in the intestines. The first major barrier to the active substance of an alcoholic beverage, which is ethanol, in the circulatory system is the liver, which literally filters the blood.
Ethanol is formed in the body, even if you do not drink alcohol: as a result of the utilization of harmful and toxic acetaldehyde by enzymes, which is a breakdown product of certain substances. But when the extra ethanol comes in with alcohol, it breaks that pattern. And the reverse reaction begins: excess ethanol, under the influence of the same enzymes, is partially converted into acetaldehyde. Both ethanol and acetaldehyde become too much.
When you drink the first glass, the liver begins to work hard: it does not know how intelligent and restrained you are, whether you stop or get drunk.
Doctors have come up with a way to "drink with a jump" Let's say you have an event in the evening - a wedding, a funeral, a birthday, where you have to drink. You are getting ready - you drink a glass of cognac during the day, and the liver begins to drive the enzyme in reserve. And when in the evening you begin to apply again, the liver triumphantly says to its cells: oh, how great that we are prepared! Circle after circle, blood and alcohol will seep through the liver filters. We pour, they speed up the work. But if a completely unbridled infusion comes, then the liver will give up. Then you will pee with pure ethanol.
(c) (extract from the article by G. Kostina, journal "Expert", No. 46)
Ethanol - what is this substance? What is its use and how is it produced? Ethanol is better known to everyone under a different name - alcohol. Of course, this is not quite the correct designation. But meanwhile, it is under the word "alcohol" that we mean "ethanol". Even our ancestors knew about its existence. They obtained it through a fermentation process. Various products from cereals to berries were used. But in the resulting Braga, which is what alcoholic drinks were called in the old days, the amount of ethanol did not exceed 15 percent. Pure alcohol could be isolated only after the distillation processes were studied.
Ethanol - what is it?
Ethanol is a monohydric alcohol. Under normal conditions, it is a volatile, colorless, flammable liquid with a specific odor and taste. Ethanol has found wide application in industry, medicine and everyday life. It is an excellent disinfectant. Alcohol is used as a fuel and as a solvent. But most of all, the formula of ethanol C2H5OH is known to lovers of alcoholic beverages. It is in this area that this substance has found wide application. But do not forget that alcohol as an active ingredient in alcoholic beverages is a strong depressant. This psychoactive substance can depress the central nervous system and cause strong dependence.
Nowadays it is difficult to find an industry where ethanol would not be used. It is difficult to list everything that alcohol is so useful for. But most of all, its properties were appreciated in pharmaceuticals. Ethanol is the main component of almost all medicinal tinctures. Many "grandmother's recipes" for the treatment of human ailments are based on this substance. It draws all useful substances from plants, accumulating them. This property of alcohol has found application in the manufacture of homemade herbal and berry tinctures. And although these are alcoholic beverages, in moderation they bring health benefits.
The benefits of ethanol

The ethanol formula is known to everyone since school chemistry lessons. But here is the benefit of this chemical, not everyone will immediately answer. In fact, it is difficult to imagine an industry where alcohol would not be used. First of all, ethanol is used in medicine as a powerful disinfectant. They treat the operating surface and wounds. Alcohol has a detrimental effect on almost all groups of microorganisms. But ethanol is used not only in surgery. It is indispensable for the manufacture of medicinal extracts and tinctures.
In small doses, alcohol is beneficial to the human body. It helps to thin the blood, improve blood circulation and dilate blood vessels. It is even used to prevent cardiovascular disease. Ethanol helps to improve the functioning of the gastrointestinal tract. But only in really small doses.
In special cases, the psychotropic effect of alcohol can drown out the most severe pains. Ethanol has found application in cosmetology. Due to its pronounced antiseptic properties, it is included in almost all cleansing lotions for problematic and oily skin.
The harm of ethanol
Ethanol is an alcohol produced by fermentation. With excessive use, it can cause severe toxicological poisoning and even coma. This substance is part of alcoholic beverages. Alcohol causes the strongest psychological and physical dependence. Alcoholism is considered to be a disease. The harm of ethanol is immediately associated with scenes of rampant drunkenness. Excessive consumption of drinks containing alcohol leads not only to food poisoning. Everything is much more complicated. With frequent drinking of alcohol, almost all organ systems are affected. From oxygen starvation, which causes ethanol, brain cells die in large numbers. Occurs In the early stages, memory weakens. Then a person develops diseases of the kidneys, liver, intestines, stomach, blood vessels and heart. In men, there is a loss of potency. In the last stages of the alcoholic, a deformation of the psyche is revealed.
History of alcohol

Ethanol - what is this substance and how was it obtained? Not everyone knows that it has been used since prehistoric times. He was part of the alcoholic beverages. True, its concentration was small. But meanwhile, traces of alcohol have been found in China on 9,000-year-old pottery. This clearly indicates that people in the Neolithic era drank alcohol-containing drinks.
The first case was recorded in the 12th century in Salerno. True, it was a water-alcohol mixture. Pure ethanol was isolated by Johann Tobias Lovitz in 1796. He used the activated carbon filtration method. For a long time, the production of ethanol by this method remained the only method. The formula for alcohol was calculated by Nicolo-Théodore de Saussure, and described as a carbon compound by Antoine Lavoisier. In the 19th and 20th centuries, many scientists studied ethanol. All its properties have been studied. Currently, it has become widespread and is used in almost all spheres of human activity.
Obtaining ethanol by alcoholic fermentation
Perhaps the most famous way to produce ethanol is alcoholic fermentation. It is possible only when using organic products that contain a large amount of carbohydrates, such as grapes, apples, berries. Another important component for fermentation to proceed actively is the presence of yeast, enzymes and bacteria. Processing of potatoes, corn, rice looks the same. To obtain fuel alcohol, raw sugar is used, which is produced from cane. The reaction is quite complex. As a result of fermentation, a solution is obtained that contains no more than 16% ethanol. A higher concentration cannot be obtained. This is due to the fact that yeast is not able to survive in more saturated solutions. Thus, the resulting ethanol must be subjected to purification and concentration processes. Usually distillation processes are used.
To obtain ethanol, use the type of yeast Saccharomyces cerevisiae of various strains. In principle, all of them are able to activate this process. As a nutrient substrate, sawdust can be used or, alternatively, a solution obtained from them.
Fuel

Many people know about the properties that ethanol has. That it is alcohol or a disinfectant is also widely known. But alcohol is also a fuel. It is used in rocket engines. A well-known fact - during the First World War, 70% aqueous ethanol was used as fuel for the world's first German ballistic missile - V-2.
Currently, alcohol has become more widespread. As a fuel, it is used in internal combustion engines, for heating devices. In laboratories, it is poured into alcohol lamps. The catalytic oxidation of ethanol is used for the production of heating pads, both military and tourist. Restricted alcohol is used in a mixture with liquid petroleum fuels due to its hygroscopicity.
Ethanol in the chemical industry
Ethanol is widely used in the chemical industry. It serves as a raw material for the production of substances such as diethyl ether, acetic acid, chloroform, ethylene, acetaldehyde, tetraethyl lead, ethyl acetate. In the paint and varnish industry, ethanol is widely used as a solvent. Alcohol is the main ingredient in windscreen washer and antifreeze. Alcohol is also used in household chemicals. It is used in detergents and cleaners. It is especially common as a component in liquids for the care of plumbing and glass.
Ethyl alcohol in medicine

Ethyl alcohol can be attributed to antiseptics. It has a detrimental effect on almost all groups of microorganisms. It destroys the cells of bacteria and microscopic fungi. The use of ethanol in medicine is almost universal. This is an excellent drying and disinfecting agent. Due to its tanning properties, alcohol (96%) is used to treat operating tables and surgeon's hands.
Ethanol is a solvent for drugs. It is widely used for the manufacture of tinctures and extracts from medicinal herbs and other plant materials. The minimum concentration of alcohol in such substances does not exceed 18 percent. Ethanol is often used as a preservative.
Ethyl alcohol is also excellent for rubbing. During a fever, it produces a cooling effect. Very often alcohol is used for warming compresses. At the same time, it is absolutely safe, there is no redness and burns on the skin. In addition, ethanol is used as a defoamer when oxygen is supplied artificially during lung ventilation. Alcohol is also a component of general anesthesia, which can be used in case of a shortage of medicines.
Oddly enough, but medical ethanol is used as an antidote for poisoning with toxic alcohols, such as methanol or ethylene glycol. Its action is due to the fact that in the presence of several substrates, the enzyme alcohol dehydrogenase performs only competitive oxidation. It is due to this that after the immediate intake of ethanol, after toxic methanol or ethylene glycol, a decrease in the current concentration of metabolites poisoning the body is observed. For methanol it is formic acid and formaldehyde, and for ethylene glycol it is oxalic acid.
food industry
So, how to get ethanol was known to our ancestors. But it was most widely used only in the 19th and 20th centuries. Along with water, ethanol is the basis of almost all alcoholic beverages, primarily vodka, gin, rum, cognac, whiskey, and beer. In small quantities, alcohol is also found in drinks that are obtained by fermentation, for example, in kefir, koumiss, and kvass. But they are not classified as alcohol, since the concentration of alcohol in them is very low. Thus, the content of ethanol in fresh kefir does not exceed 0.12%. But if it settles, then the concentration can rise to 1%. There is slightly more ethyl alcohol in kvass (up to 1.2%). Most of all alcohol is contained in koumiss. In a fresh dairy product, its concentration is from 1 to 3%, and in a settled one it reaches 4.5%.
Ethyl alcohol is a good solvent. This property allows it to be used in the food industry. Ethanol is a solvent for fragrances. In addition, it can be used as a preservative for baked goods. It is registered as a food additive E1510. Ethanol has an energy value of 7.1 kcal/g.
The effect of ethanol on the human body

Ethanol production has been established all over the world. This valuable substance is used in many areas of human life. are medicine. Wipes impregnated with this substance are used as a disinfectant. But what effect does ethanol have on our body when ingested? Is it helpful or harmful? These issues require detailed study. Everyone knows that mankind has consumed alcoholic beverages for centuries. But only in the last century the problem of alcoholism has acquired large-scale dimensions. Our ancestors drank mash, mead, and even the now so popular beer, but all of these drinks contained a low percentage of ethanol. Therefore, they could not cause significant harm to health. But after Dmitry Ivanovich Mendeleev diluted alcohol with water in certain proportions, everything changed.
Currently, alcoholism is a problem in almost all countries of the world. Once in the body, alcohol has a pathological effect on almost all organs without exception. Depending on the concentration, dose, route of entry and duration of exposure, ethanol can exhibit toxic and narcotic effects. It is able to disrupt the functioning of the cardiovascular system, contributes to the occurrence of diseases of the digestive tract, including stomach and duodenal ulcers. Under the narcotic effect is meant the ability of alcohol to cause stupor, insensitivity to pain and inhibition of the functions of the central nervous system. In addition, a person has alcoholic excitement, very quickly he becomes addicted. In some cases, excessive consumption of ethanol can cause coma.
What happens in our body when we drink alcohol? The ethanol molecule is capable of damaging the central nervous system. Under the influence of alcohol, the hormone endorphin is released in the nucleus accumbens, and in people with pronounced alcoholism and in the orbitofrontal cortex. But, nevertheless, despite this, ethanol is not recognized as a narcotic substance, although it shows all the corresponding actions. Ethyl alcohol was not included in the international list of controlled substances. And this is a controversial issue, because in certain doses, namely 12 grams of a substance per 1 kilogram of body weight, ethanol leads first to acute poisoning, and then to death.
What diseases does ethanol cause?

The ethanol solution itself is not a carcinogen. But its main metabolite, acetaldehyde, is a toxic and mutagenic substance. In addition, it also has carcinogenic properties and provokes the development of cancer. Its qualities were studied in laboratory conditions on experimental animals. These scientific works have led to very interesting, but at the same time alarming results. It turns out that acetaldehyde is not just a carcinogen, it can damage DNA.
Long-term use of alcoholic beverages can cause diseases such as gastritis, cirrhosis of the liver, duodenal ulcer, cancer of the stomach, esophagus, small and rectum, and cardiovascular diseases in humans. Regular ingestion of ethanol in the body can provoke oxidative damage to brain neurons. As a result of damage, they die. Abuse of drinks containing alcohol leads to alcoholism and clinical death. People who regularly drink alcohol have a higher risk of heart attack and stroke.
But this is not all the properties of ethanol. This substance is a natural metabolite. In small quantities, it can be synthesized in the tissues of the human body. It is called true. It is also produced as a result of the breakdown of carbohydrate foods in the gastrointestinal tract. Such ethanol is called "conditionally endogenous alcohol". Can an ordinary breathalyzer determine the alcohol that was synthesized in the body? Theoretically, this is possible. Its amount rarely exceeds 0.18 ppm. This value is at the lower limit of the most modern measuring instruments.
Ethyl alcohol С2Н5ОН (ethanol, ethyl alcohol, wine alcohol) is a colorless, volatile liquid with a characteristic odor, burning in taste (pl. 0.813-0.816, b.p. 77-77.5 ° C). Ethyl alcohol burns with a bluish flame, mixes in all proportions with water, diethyl ether and many other organic solvents, distills with water vapor.
Ethyl alcohol is obtained by fermentation of starch-containing products (grain, potatoes), fruits, sugar, etc. The ethyl alcohol obtained by fermentation is distilled off and raw alcohol is obtained, which is purified by rectification. Raw alcohol and moonshine made at home contain a certain amount of fusel oils, the composition and properties of which are described below (see Chapter IV, § 10). Fusel oils are relatively slowly metabolized in the body. Therefore, the duration of their action on the body is greater than that of ethyl alcohol.
The use of ethyl alcohol. Ethyl alcohol is widely used in industry as a solvent and starting product for the production of many chemical compounds. This alcohol is used in medicine as a disinfectant.
In chemical laboratories, it is used as a solvent, is part of many alcoholic beverages.
Effects on the body and toxicity. Ethyl alcohol can enter the body in several ways: by ingestion, by intravenous administration, and also through the lungs in the form of vapors with inhaled air.
Entered into the body of ethyl alcohol acts on the cerebral cortex. In this case, intoxication occurs with a characteristic alcoholic "excitation". This excitation is not the result of an intensification of the excitatory process, but arises from a weakening of the inhibition process. Thus, under the influence of alcohol, the predominance of excitatory processes over inhibition processes is manifested. In large doses, ethyl alcohol causes inhibition of the functions of both the spinal cord and the medulla oblongata. In this case, a state of prolonged deep anesthesia may occur with loss of reflexes and depression of vital centers. Under the influence of ethyl alcohol, death can occur as a result of paralysis of the respiratory center.
The toxicity of ethyl alcohol is evidenced by the presence of cases of acute poisoning with this alcohol. In the last decade, acute poisoning with ethyl alcohol occupies the first place (about 60%) among poisonings with other toxic substances. Alcohol not only causes acute poisoning, but also contributes to sudden death from other diseases (primarily from diseases of the cardiovascular system),
The degree of toxicity of ethyl alcohol depends on the dose, its concentration in drinks, on the presence of fusel oils and other impurities in them, added to give the drinks a certain smell and taste. Approximately lethal dose for humans is considered to be 6-8 ml of pure ethyl alcohol per 1 kg of body weight. In terms of the entire body weight, this is 200-300 ml of ethyl alcohol. However, this dose may vary depending on the sensitivity to ethyl alcohol, the conditions of its intake (the strength of drinks, the fullness of the stomach with food), etc. In some individuals, death may occur after taking 100-150 g of pure ethyl alcohol, while in other persons. death does not occur even after taking 600-800 g of this alcohol.
Prolonged abuse of ethyl alcohol leads to chronic poisoning (alcoholism). Repeated intake of alcohol leads to the development of addiction, as a result of which small doses of this alcohol cease to cause the previous euphoric state. To induce a euphoric state, such individuals require an increased dose of ethyl alcohol over time. Simultaneously with addiction, addiction is developed, and then alcohol dependence (alcoholism) develops, which is characterized by painful experiences without drinking alcohol and a strong desire to repeat its intake.
As a result of long-term use of ethyl alcohol, a number of severe violations of the body's functions occur: cirrhosis of the liver, degeneration of the heart muscle and kidneys, persistent expansion of the vessels of the face (especially the vessels of the nose), muscle trembling, hallucinations, violent delirium (delusional tremens), degeneration of male and female sex glands, as a result of which children with mental and physical insufficiency are born from alcoholics. In addition, alcohol intoxication is often the cause of accidents at home, at work, transport, etc. A significant number of violations of socialist legality and crimes are committed while intoxicated.
Thus, alcoholism is a great social evil that must be fought resolutely.
distribution in the body. Ethyl alcohol is unevenly distributed in tissues and body fluids. It depends on the amount of water in the organ or biological fluid. The quantitative content of ethyl alcohol is directly proportional to the amount of water and inversely proportional to the amount of adipose tissue in the body. The body contains about 65% of water from the total body weight. Of this amount, 75-85% of the water is contained in whole blood. Given the large volume of blood in the body, it accumulates a much larger amount of ethyl alcohol than in other organs and tissues. Therefore, the determination of ethyl alcohol in the blood is of great importance for assessing the amount of this alcohol that has entered the body. There is a definite relationship between the amount of ethyl alcohol in the blood and urine. In the first 1-2 hours after taking ethyl alcohol (alcoholic beverages), its concentration in the urine is slightly lower than in the blood. During the period of elimination, the content of ethyl alcohol in the urine taken by a catheter from the ureter exceeds its content in the blood. These data are of great importance for establishing the time elapsed from the moment of taking ethyl alcohol to the moment of the study.
Of great importance in the diagnosis of intoxication and poisoning with ethyl alcohol are the results of the quantitative determination of this alcohol, which are expressed in ppm (% 0), which means a thousandth.
When evaluating the results of the quantitative determination of ethyl alcohol in the blood, it must be taken into account that this alcohol can be formed during the putrefactive decomposition of corpses. When rotting in the blood of corpses, from negligible amounts up to 2.4 ° / o of ethyl alcohol can be formed. In the first 2-3 days after death, ethyl alcohol decomposes to a certain extent under the influence of alcohol dehydrase, which at this time still retains enzymatic activity.
Unlike blood in the urine of corpses, the formation of ethyl alcohol does not occur. Therefore, to assess the degree of intoxication, ethyl alcohol is determined both in the blood and in the urine.
Conclusions about the degree of intoxication and fatal poisoning with ethyl alcohol are made on the basis of the results of determining this alcohol in the blood. If less than 0.3 ° / oo of ethyl alcohol is found in the blood, it is concluded that this alcohol has no effect on the body. Light intoxication is characterized by the presence of 0.5-1.5% ethyl alcohol in the blood. With moderate intoxication, 1.5-2.5% 0 is found in the blood, and with severe intoxication, 2.5-3.0% of ethyl alcohol. In severe poisoning, the blood contains 3-5% b, and in fatal poisoning - 5-6% o ethyl alcohol.
Metabolism. Part of ethyl alcohol (2-10 ° / o) is excreted from the body unchanged with urine, exhaled air, sweat, saliva, feces, etc. The rest of this alcohol is metabolized. Moreover, the metabolism of ethyl alcohol can occur in several ways. A certain amount of ethyl alcohol is oxidized to form water and carbon monoxide (IV). A slightly larger amount of this alcohol is oxidized to acetaldehyde and then to acetic acid.
If antabuse, cyamide and some other substances are introduced into the body, then there is a delay in the conversion of acetaldehyde to acetic acid. This leads to the accumulation of acetaldehyde in the body, which causes an aversion to alcohol.
Ethyl alcohol detection
When examining the organs of corpses (stomach with contents, liver, kidneys, etc.) for the presence of ethyl alcohol, it is distilled off with water vapor. Ethyl alcohol is detected using the reactions described below. To detect ethyl alcohol in blood and urine, gas-liquid chromatography is used.
Microdiffusion method. Ethyl alcohol can be detected by the microdiffusion method described above (see Chapter III, § 3).
Iodoform formation reaction. When ethyl alcohol is heated with a solution of iodine and alkali, iodoform (SH3) is formed, which has a specific smell:
Execution of the reaction. Add 1 ml of the test solution and 2 ml of a 5% solution of sodium hydroxide or sodium carbonate to the test tube. A 1% solution of iodine in a 2% solution of potassium iodide is added dropwise to this mixture until a slightly yellow color is obtained. The mixture is then heated for several minutes in a water bath (50°C). In the presence of ethyl alcohol, the smell of iodoform is felt. At relatively large amounts of ethyl alcohol, iodoform crystals are formed in the sample, having the shape of hexagons and stars.
Limit of detection: 0.04 mg of ethyl alcohol in 1 ml of solution. This reaction is not specific to ethyl alcohol. It is given by acetone, lactic acid, etc.
esterification reaction. Sodium acetate and benzoyl chloride are used to esterify ethyl alcohol.
1. The reaction of the formation of acetic ethyl ester. Ethyl alcohol with sodium acetate in the presence of sulfuric acid forms acetic ethyl ester, which has a characteristic odor:
Execution of the reaction. 1 ml of the test solution and 0.1 g of dried sodium acetate are added to the test tube, then 2 ml of concentrated sulfuric acid are carefully added dropwise. The mixture is heated on a burner flame (it is better to heat the test tube in a paraffin or glycerol bath) until gas bubbles are released. The appearance of a specific smell of ethyl acetate indicates the presence of ethyl alcohol in the test solution.
Limit of detection: 15 µg of ethyl alcohol in 1 ml of solution.
The smell of acetic-ethyl ether is more clearly felt if the contents of the test tube are poured into 20-25 times the volume of water.
2. The reaction of the formation of ethyl benzoate. When ethyl alcohol reacts with benzoyl chloride (benzoyl chloride), ethyl benzoate is formed, which has a characteristic odor:
Recognition of the smell of ethyl benzoate is hindered by an excess of benzoyl chloride, which has an unpleasant odor. Therefore, to decompose an excess of benzoyl chloride, an alkali solution is added:
Execution of the reaction. To 1 ml of the test solution add 1-2 drops of benzoyl chloride. With frequent agitation of the mixture, a 10% solution of sodium hydroxide is added dropwise to it until the suffocating smell of benzoyl chloride disappears. The appearance of an odor of ethyl benzoate indicates the presence of ethyl alcohol in the sample. This smell is better, felt after applying a few drops of the reaction mixture to a piece of filter paper. The reaction is hindered by methyl alcohol, since the smell of ethyl benzoate resembles the smell of benzoic methyl ether.
The reaction of the formation of acetaldehyde. Ethyl alcohol is oxidized with potassium dichromate, potassium permanganate and some other oxidizing agents to acetaldehyde:
Execution of the reaction. A 10% solution of sulfuric acid is added to 1 ml of the test solution until an acidic medium is obtained (according to litmus). A 10% solution of potassium dichromate is added dropwise to this mixture until the liquid becomes orange-red. The mixture is left for several minutes at room temperature. In the presence of ethyl alcohol in the test solution, the smell of acetaldehyde appears. This reaction may also produce some acetic acid. The side reaction of acetic acid formation reduces the sensitivity of the acetaldehyde detection reaction.
Oxidation of ethyl alcohol and its detection by acetaldehyde. Acetaldehyde, formed during the oxidation of ethanol, can be detected by reaction with sodium nitroprusside and morpholine. For this purpose, 2-3 drops of a solution4 containing acetaldehyde are applied to a drip plate or filter paper and a drop of reagent is added (a freshly prepared mixture of equal volumes of a 20% aqueous solution of morpholine and a 5% aqueous solution of sodium nitroprusside). In the presence of acetaldehyde, a blue color appears in the solution.
Limit of detection: 1 µg of acetaldehyde per sample.
This reaction is given by acrolein and some other aldehydes. The reaction with morpholine and sodium nitroprusside gives propionaldehyde only at high concentrations. Formaldehyde does not give this reaction. Therefore, the oxidation reaction of ethyl alcohol to acetaldehyde and its detection with morpholine and sodium nitroprusside can be used to distinguish between methyl and ethyl alcohols.
Preliminary test for the presence of ethyl alcohol in urine and blood. This test is described in detail above (see Chapter IV, § 8).
Detection of ethyl alcohol in drinks and solutions by gas-liquid chromatography
The principle of detecting chemical compounds using the gas-liquid chromatography method is described in a number of literature sources. To detect ethyl alcohol in solutions, drinks and other liquids by gas-liquid chromatography, 95% ethyl alcohol is used as a reference substance. Before being introduced into the gas chromatograph dispenser, this alcohol is converted into a more volatile compound than ethyl alcohol (bp 78 ° C), the compound is ethyl nitrite (bp 17 ° C). To do this, sodium or potassium nitrite and trichloroacetic acid are added to ethyl alcohol:
The resulting ethyl nitrite, which is in a gaseous state above the liquid, is introduced into a gas chromatograph and chromatography is performed.
Chromatography conditions:
a chromatograph equipped with a katharometer;
metal column 100 cm long, 0.6 cm in diameter;
solid carrier: spherochrome, hesasorb or other carriers;
stationary liquid phase: polyethylene glycol (mol. weight 1000-1500) deposited on a solid carrier in an amount of 12%;
the temperature of the thermostats of the column and the detector is 75 °С, the temperature of the dispenser is room temperature;
carrier gas: technical nitrogen passed through the chromatograph at a rate of 50-60 ml/min;
detector current 60-100 mA;
chart tape speed 720 mm/h.
Method for the detection of ethyl alcohol in drinks and solutions. 0.5 ml of a 50% solution of trichloroacetic acid and 0.5 ml of an aqueous solution of a reference substance (95% ethyl alcohol diluted with water so that its concentration is 3-4% o) are added to a penicillin vial. ). The bottle is closed with a rubber stopper, which is fixed with a special device (clamp). Then, using a syringe through a rubber stopper, 0.25 ml of a 30% sodium nitrite solution is injected into the vial. The contents of the vial are shaken well for 1 min and 3 ml of the gaseous phase above the liquid is collected using another dry syringe. This gaseous phase containing ethyl nitrite is introduced into the chromatograph dispenser and chromatographed. While recording the retention time of ethyl nitrite.
After chromatography of the reference substance is completed, exactly the same experiment is performed with the test solution, in which the presence of ethyl alcohol is assumed.
The coincidence of the retention time of the substance in both samples (in the sample with the reference and test substances) indicates the identity of these substances.
Method for detecting ethyl alcohol in blood and urine. The method for detecting ethyl alcohol in blood and urine is similar to the method for detecting this alcohol in drinks and solutions. First, chromatography is performed and the retention time of ethyl alcohol, which is the reference substance, is determined. This determination is made as indicated in the description of the method for determining this alcohol in drinks and solutions. Then proceed to the determination of ethyl alcohol in the blood or urine.
0.5 ml of the test blood or urine and 0.5 ml of a 50% solution of trichloroacetic acid are added to the penicillin vial. The bottle is closed with a rubber stopper, which is fixed with a special fixative. After that, 0.25 ml of a 30% sodium nitrite solution is injected into the vial through a rubber stopper using a syringe. The contents of the vial are well shaken for one minute. Then, 3 ml of the gaseous phase is taken from the vial with another syringe, which is injected into the chromatograph dispenser and chromatographed. If the retention time of the reference substance and the substance contained in the blood or urine coincide, a conclusion is made about the presence of ethyl alcohol in the studied biological fluids.
If ethyl alcohol is detected in the urine or in the blood, the method of gas-liquid chromatography produces a quantitative determination of this alcohol in these objects.
Quantitative determination of ethyl alcohol in blood and urine by gas-liquid chromatography
For the quantitative determination of ethyl alcohol in blood and urine, the internal standard method is used, as one of the methods of gas-liquid chromatography. According to this method, an internal standard is added to the blood or urine, in which the quantitative content of ethyl alcohol is determined. Propyl alcohol is used as an internal standard. The ethyl alcohol contained in the blood or urine (bp 78 ° C), as well as propyl alcohol (bp 97.5 ° C), added as an internal standard, are converted into more volatile compounds (into ethyl nitrite with t. bp 17°C and propyl nitrite bp 46-48°C). A mixture of ethyl nitrite and propyl nitrite is introduced into the chromatograph dispenser and chromatography is carried out. In this case, two peaks are written on the chromatogram, one of which corresponds to ethyl alcohol (ethyl nitrite), and the second to propyl alcohol (propyl nitrite). Then the ratio of the area or height of the peak of ethyl alcohol (ethyl nitrite) to the area or height of the peak of the internal standard - propyl alcohol (propyl nitrite) is calculated.
The calculation of the quantitative content of ethyl alcohol in the blood or urine is carried out according to the calibration curve.
Construction of a calibration graph. First, prepare a series of standard solutions containing 2, 3, 4 and 5% ethanol, and an internal standard solution containing 4% 0. propyl alcohol. In several vials of penicillin, 2 ml of a solution containing 4% 0 propyl alcohol is added. 2 ml of ethyl alcohol solution of various concentrations (2, 3, 4 and 5% o) are added to each vial. The contents of the vials are mixed well, and then 1 ml of a mixture of alcohols is taken from each vial and transferred to other penicillin vials. Add 0.5 ml of 50°/o-th solution of trichloroacetic acid to each vial. The vials are closed with rubber stoppers, which are fixed with clamps. Then, using a syringe through a rubber stopper, 0.25 ml of a 30% solution of sodium nitrite is introduced into the vials. The contents of the vials are shaken for one minute. After that, with the help of another dry syringe, 3 ml are taken from the vials
The gaseous phase, which is introduced into the chromatograph dispenser, and chromatographed.
Chromatography conditions are indicated above in the description of the method for detecting ethyl alcohol in drinks and solutions.
Chromatograms measure the area or height of each peak. Then find the ratio of the area or height of the peak of ethyl alcohol (ethyl nitrite) to the area or height of the peak of the internal standard (propyl nitrite). Taking into account that in this case for different concentrations of ethanol, values are obtained that differ slightly from each other, they are multiplied by 100 and the multiplication results are plotted on the y-axis of the calibration graph. On the abscissa of the calibration graph put the value of the concentration of ethyl alcohol (in ° / oo).
Determination of ethyl alcohol in blood and urine. 2 ml of an internal standard solution (propyl alcohol, the concentration of which is 4%) is added to the penicillin vial, 2 ml of blood or urine to be examined for the presence of ethyl alcohol is added. The contents of the vial are shaken well, and then 1 ml of liquid (a mixture of blood or urine with an internal standard) is transferred to another vial from penicillin and 0.5 ml of a 50% solution of trichloroacetic acid is added. The bottle is closed with a rubber stopper, which is fixed with a fixative. Using a syringe, 0.25 ml of a 30% sodium nitrite solution is introduced into the vial through a stopper. The contents of the vial are shaken for one minute. Then, using another dry syringe, 3 ml of the gaseous phase is taken from the vial, which is transferred to the chromatograph dispenser, and chromatography is carried out.
Peak areas or heights are determined on the chromatogram and the ratio of the area or height of the ethanol peak to the area or peak height of the internal standard is calculated. Based on this ratio, multiplied by 100, the ethanol content in the blood or urine (in %) is calculated from the calibration curve.
When determining ethyl alcohol in the blood, the concentration of this alcohol found from the calibration graph is multiplied by 0.95, and the concentration of ethyl alcohol found in the urine is multiplied by 1.05.